Human VEGFR2/KDR/Flk-1 PE-conjugated Antibody
R&D Systems, part of Bio-Techne | Catalog # FAB357P


Key Product Details
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
Ala20-Glu764
Accession # P35968
Specificity
Clonality
Host
Isotype
Scientific Data Images for Human VEGFR2/KDR/Flk-1 PE-conjugated Antibody
Detection of VEGF R2/KDR/Flk‑1 in HUVEC Human Cells by Flow Cytometry.
HUVEC human umbilical vein endothelial cells were stained with Mouse Anti-Human VEGF R2/KDR/Flk-1 PE-conjugated Mono-clonal Antibody (Catalog # FAB357P, filled histogram) or isotype control antibody (Catalog # IC002P, open histogram). View our protocol for Staining Membrane-associated Proteins.Detection of Human VEGFR2/KDR/Flk-1 by Flow Cytometry
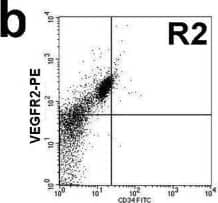
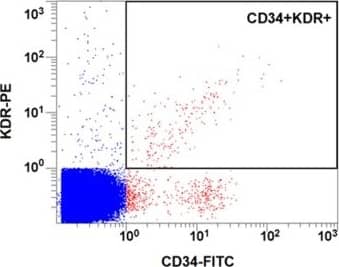
Circulating EPC number in healthy controls and diabetes. a and b: Circulating EPC numbers were determined by flow cytometry for the co-expression of CD34 and VEGFR2 (b). Peripheral blood MNCs incubated with IgG isotype control (a) serve as a negative control to determine the intrinsic fluorescent intensity of the peripheral blood MNCs and to define positive area R2. c: Absolute number of circulating EPCs in healthy controls and diabetes as determined by the co-expression of CD34 and VEGFR2. d: Absolute number of circulating EPCs in healthy controls, diabetes with good and poor glycemic control as determined by the co-expression of CD34 and VEGFR2. e: Correlation between the circulating EPC numbers and FBS, f: Correlation between the circulating EPC numbers and HbA1C, g: Absolute number of circulating CD34+/VEGFR2- cells in healthy controls and diabetes. Data are presented as means ± SEM. Image collected and cropped by CiteAb from the following publication (https://bmcendocrdisord.biomedcentral.com/articles/10.1186/1472-6823-10-5), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of Human VEGFR2/KDR/Flk-1 by Flow Cytometry
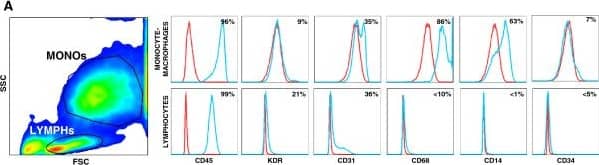
Phenotypic characterization of cultured PACs. A) PACs were characterized for surface marker expression by flow cytometry. In the side scatted (SSC) versus forward scatter (FSC) morphologic plot, lymphocytic cells (LYMPHs) and monocyte-macrophages (MONOs) were identified and gated separately. Histograms reporting the expression of relevant leukocyte (CD45, CD14, CD68) and endothelial markers (CD31, KDR, CD34) are shown, together with mean percent expression from 3 replicates. The red line indicates negative control, while the blue line indicates the stained condition. B) Cells in the PACs culture were stained with the endothelial markers acLDL and Ulex Lectin. The fraction of cells that were positive for both markers were compared in cultures obtained from T2D or healthy control cells. *p < 0.05 T2D vs Ctrl. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24886621), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Human VEGFR2/KDR/Flk-1 PE-conjugated Antibody
Flow Cytometry
Sample: HUVEC human umbilical vein endothelial cells
Reviewed Applications
Read 5 reviews rated 3.8 using FAB357P in the following applications:
Formulation, Preparation, and Storage
Purification
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, 2 to 8 °C as supplied.
Background: VEGFR2/KDR/Flk-1
VEGF R2 (KDR/Flk-1), VEGF R1 (Flt-1) and VEGF R3 (Flt-4) belong to the class III subfamily of receptor tyrosine kinases (RTKs). All three receptors contain seven immunoglobulin-like repeats in their extracellular domains and kinase insert domains in their intracellular regions. The expression of VEGF R1, 2, and 3 is almost exclusively restricted to the endothelial cells. These receptors are likely to play essential roles in vasculogenesis and angiogenesis. Mature VEGF R2 is composed of a 745 aa extracellular domain, a 25 aa transmembrane domain and a 567 aa cytoplasmic domain. In contrast to VEGF R1 which binds both PlGF and VEGF with high affinity, VEGF R2 binds VEGF but not PlGF with high affinity. The recombinant soluble VEGF R2/Fc chimera binds VEGF with high affinity and is a potent VEGF antagonist.
References
- Ferra, N. and R. Davis-Smyth (1997) Endocrine Reviews 18:4.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional VEGFR2/KDR/Flk-1 Products
Product Documents for Human VEGFR2/KDR/Flk-1 PE-conjugated Antibody
Product Specific Notices for Human VEGFR2/KDR/Flk-1 PE-conjugated Antibody
For research use only