Recombinant BatCoV RaTG13 Spike S1 Subunit His Protein, CF
R&D Systems, part of Bio-Techne | Catalog # 10662-CV

Key Product Details
Product Specifications
Source
Chinese Hamster Ovary cell line, CHO-derived batcov ratg13 Spike S1 Subunit protein
Val16-Ser680, with a C-terminal 6-His tag
Val16-Ser680, with a C-terminal 6-His tag
Purity
>95%, by SDS-PAGE visualized with Silver Staining and quantitative densitometry by Coomassie® Blue Staining.
Endotoxin Level
<0.10 EU per 1 μg of the protein by the LAL method.
N-terminal Sequence Analysis
Val 16
Predicted Molecular Mass
76 kDa
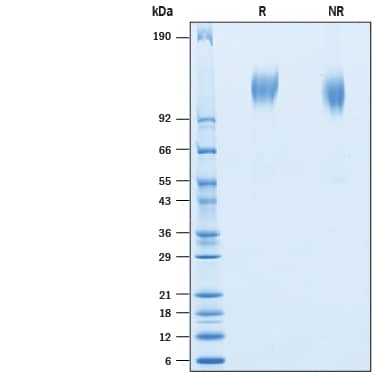
SDS-PAGE
113-127 kDa, under reducing conditions
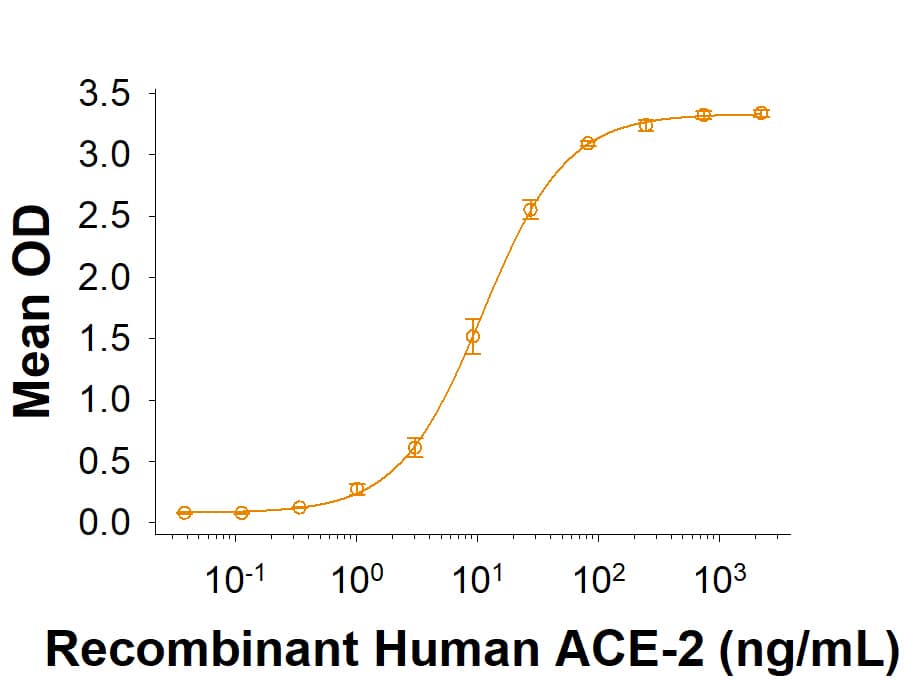
Activity
Measured by its binding ability in a functional ELISA with Recombinant
Human ACE-2 Fc Chimera
(Catalog #
10544-ZN).
Scientific Data Images for Recombinant BatCoV RaTG13 Spike S1 Subunit His Protein, CF
Recombinant BatCoV RaTG13 Spike S1 Subunit His-tag Protein Binding Activity.
Recombinant BatCoV RaTG13 Spike S1 Subunit His-tag (Catalog # 10662-CV) binds Recombinant Human ACE-2 Fc Chimera (10544-ZN) in a functional ELISA.Recombinant BatCoV RaTG13 Spike S1 Subunit His-tag Protein SDS-PAGE.
2 μg/lane of Recombinant BatCoV RaTG13 Spike S1 Subunit His-tag (Catalog # 10662-CV) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 113-127 kDa.Formulation, Preparation and Storage
10662-CV
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in PBS. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: Spike S1 Subunit
References
- Wu, F. et al. (2020) Nature 579:265.
- Tortorici, M.A. and D. Veesler (2019) Adv. Virus Res. 105:93.
- Bosch, B.J. et al. (2003). J. Virol. 77:8801.
- Belouzard, S. et al. (2009) Proc. Natl. Acad. Sci. 106:5871.
- Millet, J.K. and G. R. Whittaker (2015) Virus Res. 202:120.
- Yuan, Y. et al. (2017) Nat. Commun. 8:15092.
- Walls, A.C. et al. (2010) Cell 180:281.
- Malayia, J. et al. (2020) J Med. Virol. https://doi.org/10.1002/jmv.26261.
- Wrobel, A.G. et al. (2020) Nat. struct. Mol. Biol. https://doi.org/10.1038/s41594-020-0468-7.
- Ortega, J.T. et al. (2020) EXCLI J. 19:410.
- Wrapp, D. et al. (2020) Science 367:1260.
- Tai, W. et al. (2020) Cell. Mol. Immunol. https://doi.org/10.1016/j.it.2020.03.007.
- Okba, N. M. A. et al. (2020). Emerg. Infect. Dis. https://doi.org/10.3201/eid2607.200841.
- Wang, X. et al. (2020) https://doi.org/10.1038/s41423-020-0424-9.
- Wang, K. et al. (2020) bioRxiv https://doi.org/10.1101/2020.03.14.988345.
Long Name
Spike Protein, S1 Subunit
Alternate Names
SARS-CoV-2
UniProt
Additional Spike S1 Subunit Products
Product Documents for Recombinant BatCoV RaTG13 Spike S1 Subunit His Protein, CF
Product Specific Notices for Recombinant BatCoV RaTG13 Spike S1 Subunit His Protein, CF
For research use only
Loading...
Loading...
Loading...