Recombinant Human FGF basic Heat Stable GMP Protein, CF GMP Best Seller
R&D Systems, part of Bio-Techne | Catalog # BT-FGFBHS-GMP

Key Product Details
- FGF basic Heat Stable Manufactured in Bio-Techne's new GMP facility
- Lot-to-lot consistency
- Stringent guidelines for patient safety
- Scalability necessary to support successful therapeutics
- Learn more about manufacturing in our new GMP facility
- Test it in your process! Request a sample of GMP FGF basic Heat Stable
Product Specifications
Source
Ala135-Ser288 (with modifications)
Produced using non-animal reagents in an animal-free laboratory.Manufactured and tested under cGMP guidelines.
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
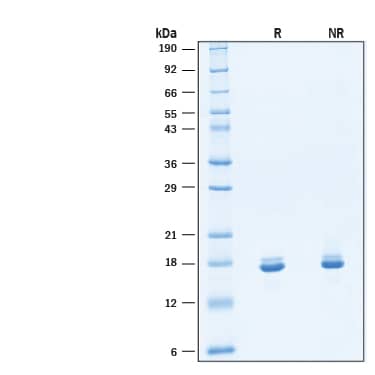
SDS-PAGE
Activity
The ED50 for this effect is 0.0500-0.600 ng/mL.
Host Cell Protein
Mycoplasma
Host Cell DNA
Scientific Data Images for Recombinant Human FGF basic Heat Stable GMP Protein, CF
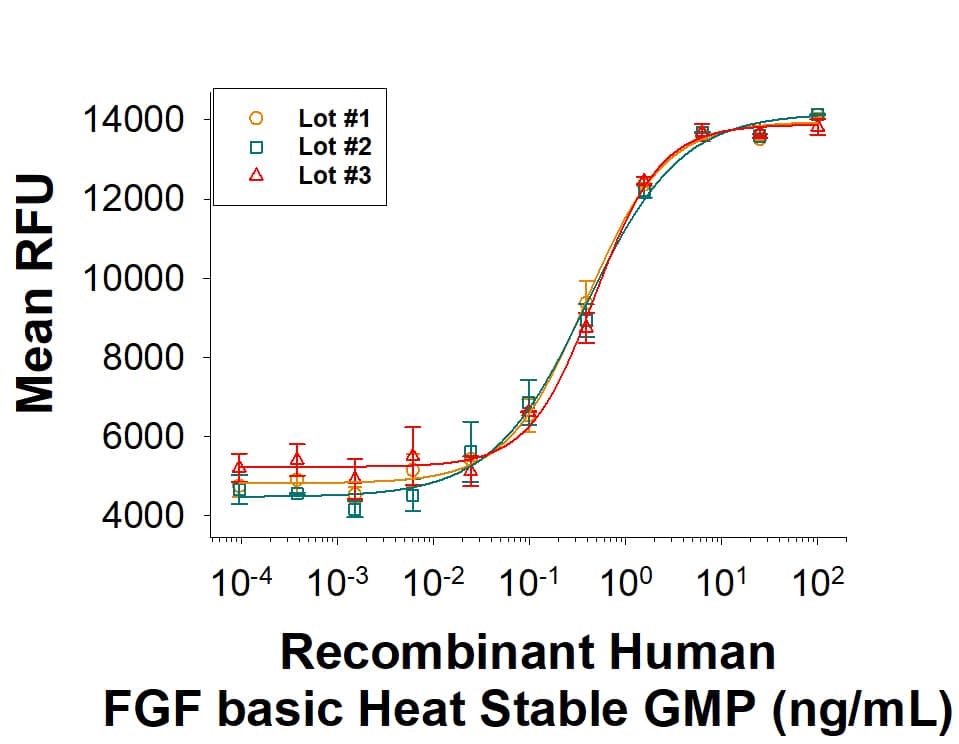
Recombinant Human FGF basic Heat Stable GMP Protein Bioactivity.
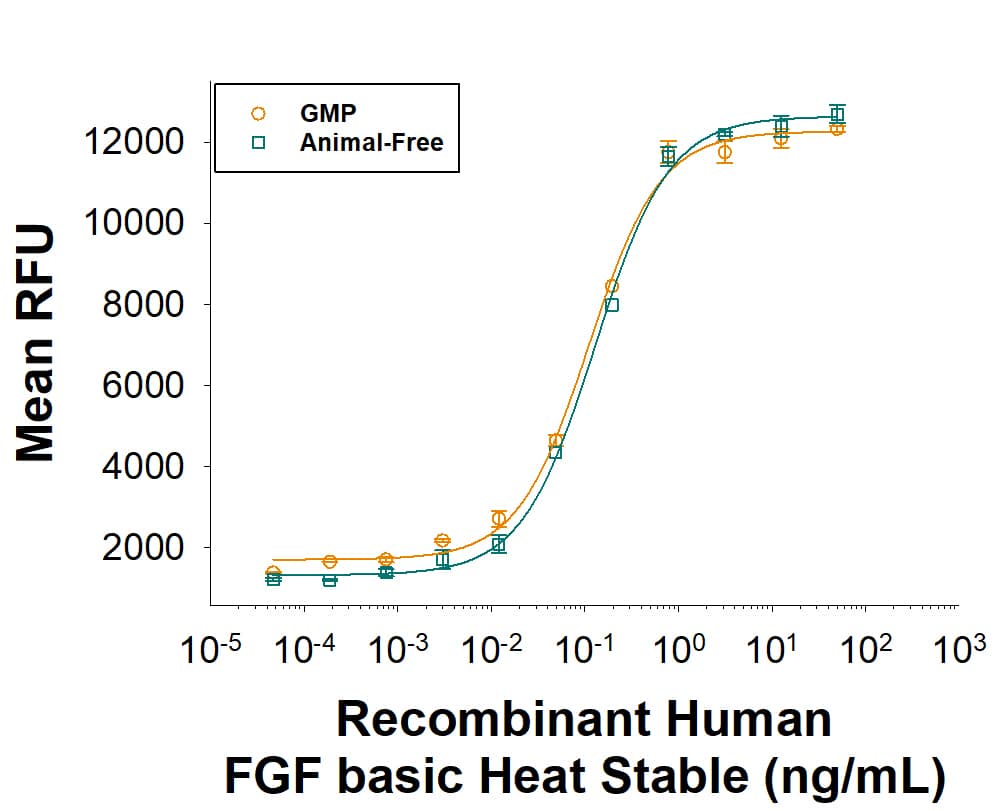
The bioactivity of Recombinant Human FGF basic Heat Stable GMP Protein (Catalog # BT-FGFBHS-GMP) was measured in a cell proliferation assay using NR6R-3T3 mouse fibroblast cell line. The ED50 for this effect is 0.0500-0.600 ng/mL. Three independent lots were tested for bioactivity and plotted on the same graph to show lot-to-lot consistency of GMP FGF basic/FGF2 Heat Stabile protein.Equivalent bioactivity of GMP and Animal-Free grades of recombinant human FGF basic heat-stable proteins.
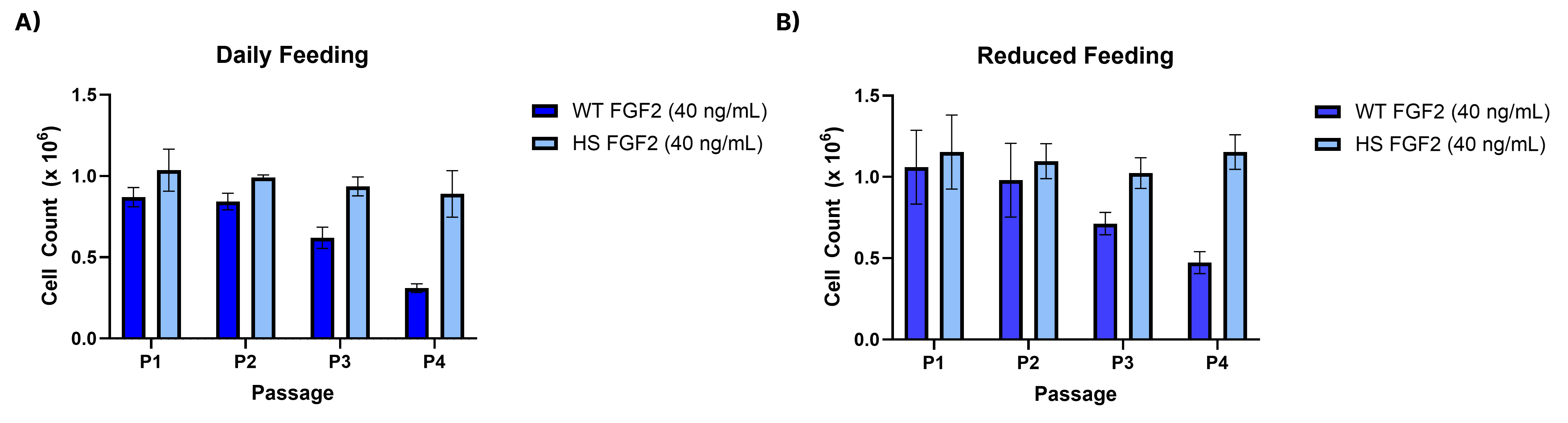
Equivalent bioactivity of GMP (BT-FGFBHS-GMP) and Animal-Free (BT-FGFBHS-AFL) grades of Recombinant Human FGF basic Heat Stable proteins as measured in a cell proliferation assay using NR6R-3T3 mouse fibroblast cells (orange and green, respectively).Higher iPSC fold expansion is observed with rhFGFb Heat Stable compared to wild type rhFGFb for daily and reduced feeding at 40 ng/mL
Human iPSCs were cultured for four passages in a commercial iPSC Expansion media containing 40 ng/mL of either rhFGFb Heat Stable (BT-FGFBHS) or wild type rhFGFb, Animal-Free (BT-FGFB-AFL). Media exchanges were performed following two feeding schedules. A). a daily feed schedule or B). a reduced feed schedule, where cells were double fed twice per week. Cells were plated at 5000 cells/cm2in a 6-well dish coated with Cultrex Ultimatrix (BME001). For each schedule, all conditions were passaged once either group reached ~60-70% confluency and counts were performed using a Cellaca™ High-Throughput Cell Counter. Count of each passage is shown comparing WT vs HS FGFb in both the A) daily feed or B) reduced feed schedule.Formulation, Preparation and Storage
BT-FGFBHS-GMP
| Formulation | Lyophilized from a 0.2 μm filtered solution in HEPES and Sodium Sulfate with Trehalose. |
| Reconstitution | Reconstitute the 25 μg size at 250 μg/mL in sterile water. Reconstitute all other sizes at 500 μg/mL in sterile water. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: FGF basic/FGF2/bFGF
Long Name
Alternate Names
Gene Symbol
UniProt
Additional FGF basic/FGF2/bFGF Products
Product Documents for Recombinant Human FGF basic Heat Stable GMP Protein, CF
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented processes and QA control of documentation and process changes
- Personnel training programs
- Raw material testing and vendor qualification/monitoring
- Fully validated equipment, processes and test methods
- Equipment calibration schedules using a computerized calibration program
- Facility maintenance, safety programs and pest control
- Material review process for variances
- Monitoring of stability over product shelf-life
- N-terminal amino acid analysis, SDS-PAGE analysis, mass spectrometry, and endotoxin level (as determined by LAL assay) performed on each bulk QC lot, not on individual bottlings of each QC lot
- Post-bottling lot-specific bioassay results (compliance with an established range) and results of microbial testing according to USP <71>
- Host Cell Protein testing performed by ELISA
- Mycoplasma testing by ribosomal RNA hybridization assay
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Animal-Free Manufacturing Conditions
Our dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers in a dedicated cold storage room.
- Low Endotoxin Level.
- No impairment of biological activity.
- High quality product obtained under stringent conditions.
Product Specific Notices for Recombinant Human FGF basic Heat Stable GMP Protein, CF
Produced under license from Enantis s.r.o.
END USER TERMS OF USE OF PRODUCT
The following terms are offered to you upon your acceptance of these End User Terms of Use of Product. By using this product, you indicate your acknowledgment and agreement to these End User Terms of Use of Product. If you do not agree to be bound by and comply with all of the provisions of these End User Terms of Use of Product, you should contact your supplier of the product and make arrangements to return the product.
We suggest you print and retain a copy of these End User Terms of Use of Product for your records.
The End User is aware that R&D Systems, Inc. sells GMP products for preclinical or clinical ex vivo use and not for in vivo use. The End User further agrees, as a condition of the sale of R&D Systems' GMP products that: a) the End User will not use this GMP Product in any procedure wherein the product may be directly or indirectly administered to humans, unless the End User has obtained, or prior to their use will have obtained, an Investigational New Drug (IND) exemption from the FDA and will use the product only in accordance with the protocols of such IND and of the Institutional Review Board overseeing the proposed research, or b) the End User will use the products outside of the United States in accordance with the protocols of research approved by the Institutional Review Board or authorized ethics committee and regulatory agencies to which the End User is subject to in their territory.
R&D Systems, Inc. has the right, at its sole discretion, to modify, add or remove any terms or conditions of these End User Terms of Use without notice or liability to you. Any changes to these End User Terms of Use are effective immediately following the printing of such changes on this product insert. The most recent version of these End User Terms of Use of Product may be found at: RnDSystems.com/Legal.
You agree to review these End User Terms of Use of Product to ensure any subsequent use by you of R&D Systems' GMP Products following changes to these End User Terms of Use of Product constitutes your acceptance of all such changes.
TERMS AND CONDITIONS
The following limitation applies to R&D Systems' warranty and liability for damages: All products are warranted to meet R&D Systems' published specifications when used under normal laboratory conditions.
R&D SYSTEMS DOES NOT MAKE ANY OTHER WARRANTY OR REPRESENTATION WHATSOEVER, WHETHER EXPRESS OR IMPLIED, WITH RESPECT TO ITS PRODUCTS. IN PARTICULAR, R&D SYSTEMS DOES NOT MAKE ANY WARRANTY OF SUITABILITY, NONINFRINGEMENT, MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE.
NOTWITHSTANDING ANY OTHER PROVISIONS OF THESE TERMS AND/OR ANY OTHER AGREEMENT BETWEEN R&D SYSTEMS AND PURCHASER FOR THE PURCHASE OF THE PRODUCTS, R&D SYSTEMS' TOTAL LIABILITY TO PURCHASER ARISING FROM OR IN RELATION TO THESE TERMS, AN AGREEMENT BETWEEN THE PARTIES OR THE PRODUCTS, WHETHER ARISING IN CONTRACT, TORT OR OTHERWISE SHALL BE LIMITED TO THE TOTAL AMOUNT PAID BY PURCHASER TO R&D SYSTEMS FOR THE APPLICABLE PRODUCTS. IN NO EVENT WILL R&D SYSTEMS BE LIABLE FOR THE COST OF PROCUREMENT OF SUBSTITUTE GOODS.
Full details of R&D Systems' Terms and Conditions of Sale can be found online at: RnDSystems.com/Legal.
For preclinical, or clinical ex vivo use