Recombinant Human O-GlcNAcTransferase/OGT Protein, CF
R&D Systems, part of Bio-Techne | Catalog # 8446-GT

Key Product Details
Learn more about Fluorescent Glycan Labeling and Detection
Product Specifications
Source
E. coli-derived human O-GlcNAc Transferase/OGT protein
Cys323-Glu1041, with C-terminal 6-His tag
Cys323-Glu1041, with C-terminal 6-His tag
Purity
>80%, by SDS-PAGE visualized with Silver Staining and quantitative densitometry by Coomassie® Blue Staining.
Endotoxin Level
<1.0 EU per 1 μg of the protein by the LAL method.
N-terminal Sequence Analysis
Cys323
Predicted Molecular Mass
81 kDa
SDS-PAGE
70 kDa, reducing conditions
Activity
Measured by its ability to transfer GlcNAc from UDP-GclNAc to peptide OGT substrate from AnaSpec, Inc.
The specific activity is >25 pmol/min/μg, as measured under the described conditions.
The specific activity is >25 pmol/min/μg, as measured under the described conditions.
Scientific Data Images for Recombinant Human O-GlcNAcTransferase/OGT Protein, CF
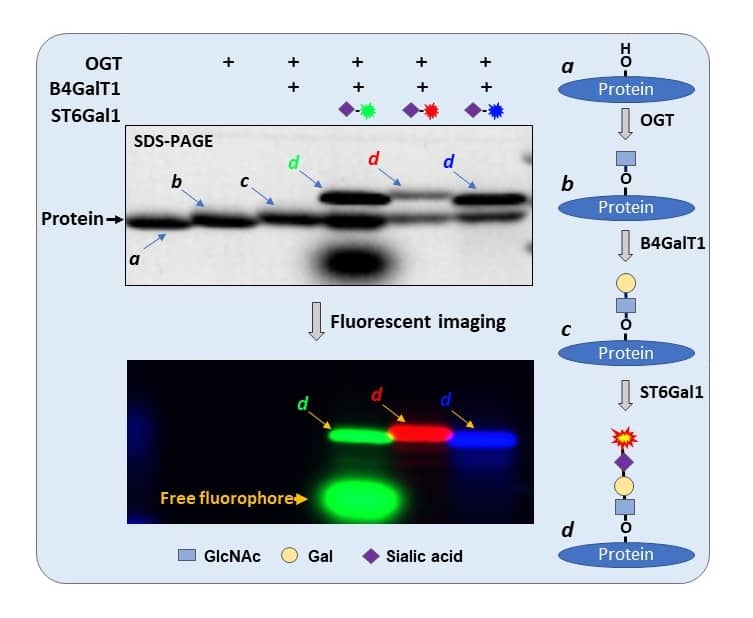
Detecting O-GlcNAc site on recombinant CK2 with different fluorophore-conjugated sialic acids.
O-GlcNAc was first introduced to Recombinant Human Casein Kinase 2 alpha Protein, CF (7957-CK) and then elongated by Recombinant Human B4GalT1 Protein, CF (3609-GT) and finally labeled by Recombinant Human ST6GAL1 (aa 44-406) Protein, CF (7620-GT) with different fluorophore-conjugated sialic acids. Samples were separated on 4-20% SDS-PAGE and visualized for protein with trichloroacetic acid imaging (upper panel) and fluorescent imaging (lower panel). From lane 4 to 6, O-GlcNAcylated rhCK2 was labeled with CMP-Cy3-Neu5Ac (d) (ES402), CMP-Cy5-Neu5Ac (d) (ES302) and CMP-AlexaFluor 488-Neu5Ac (d), respectively. Cy3 and AlexaFluor 488 have additional absorbance in TCE image, therefore resulting extra band intensities on the labeled bands. More information on O-GlcNAc labeling can be found at O-GlcNAc/O-GalNAc Labeling Reagents.O-GlcNAc Detection Using the Tandem Labeling or Direct Labeling Methods.
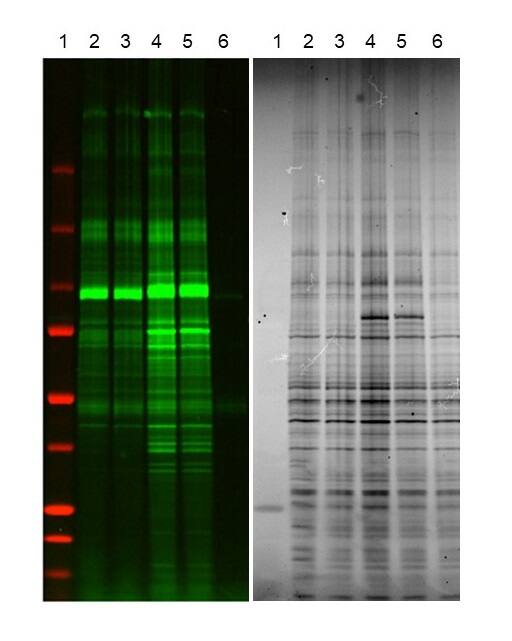
Nuclear extracts of HEK293 cells were tandem labeled on O-GlcNAc using Recombinant Human B4GalT1 Protein, CF (3609-GT) and by Recombinant Human ST6GAL1 (aa 44-406) Protein, CF (7620-GT). Lane 1 contains a protein Western marker, lanes 4 and 5 contain samples that were treated with Recombinant Human O-GlcNAc Transferase/OGT Protein, CF (Catalog # 8446-GT) first and then labeled for O-GlcNAc, lane 6 contains a sample that was labeled with Recombinant Human ST6Gal1 only. The left side of the figure is the fluorescent image and the right side of the figure is the TCE image of the gel. More information on O-GlcNAc labeling can be found at O-GlcNAc/O-GalNAc Labeling Reagents.Recombinant Human O-GlcNAc Transferase/OGT Protein Enzyme Activity Diagram.
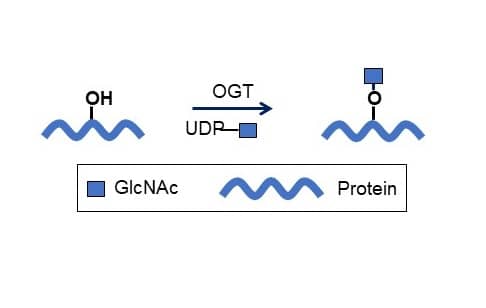
Recombinant Human O-GlcNAc Transferase/OGT Protein, CF (Catalog # 8446-GT) catalyzes the addition of a single GlcNAc in O-glycosidic linkage to serine or threonine residues.Formulation, Preparation and Storage
8446-GT
| Formulation | Supplied as a 0.2 μm filtered solution in Tris, NaCl and TCEP. |
| Shipping | The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | 6 months from date of receipt, <70 °C as supplied. 3 months, <70 °C under sterile conditions after opening. |
Background: O-GlcNAc Transferase/OGT
References
- Hart, G.W. et al. (2007) Nature 446:1017.
- Love D.C. and Hanover J.A. (2005) Sci. STKE. 2005:re13.
- Comer, F.I. and Hart, G.W. (2001) Biochemistry 40:7845.
- Hart, G.W. et al. (2011) Annu. Rev. Biochem. 80:825.
- Ma J. and Hart, GW. (2013) Expert Rev. Proteomics 10:365.
- Slawson C, et al. (2006) J. Biol. Chem. 97:71.
- Dentin, R. et al. (2008) Science 319:1402.
- Lazarus, M.B. et al. (2011) Nature 469:564.
- Wu, Z.L. et al. (2011) Glycobiology 21:727.
Long Name
O-Linked N-Acetylglucosamine (GlcNAc) Transferase
Alternate Names
HRNT1, OGlcNAc Transferase, OGT
Gene Symbol
OGT
UniProt
Additional O-GlcNAc Transferase/OGT Products
Product Documents for Recombinant Human O-GlcNAcTransferase/OGT Protein, CF
Product Specific Notices for Recombinant Human O-GlcNAcTransferase/OGT Protein, CF
For research use only
Loading...
Loading...
Loading...