Recombinant Human Thrombopoietin GMP Protein, CF GMP Best Seller
R&D Systems, part of Bio-Techne | Catalog # 288E-GMP
Animal Free

Key Product Details
Product Specifications
Source
E. coli-derived human Thrombopoietin/Tpo protein
Ser22-Leu195
Produced in an animal-free laboratory. Manufactured and tested under cGMP guidelines
Ser22-Leu195
Produced in an animal-free laboratory. Manufactured and tested under cGMP guidelines
Purity
>95%, by SDS-PAGE visualized with Silver Staining and quantitative densitometry by Coomassie® Blue Staining.
Endotoxin Level
<0.10 EU per 1 μg of the protein by the LAL method.
N-terminal Sequence Analysis
Ala-Ser22-Pro-Ala-Pro-Pro-Ala-(Cys)-Asp-Leu
Predicted Molecular Mass
18.7 kDa
SDS-PAGE
19 kDa, reducing conditions
Activity
Measured in a cell proliferation assay using MO7e human megakaryocytic leukemic cells. Avanzi, G. et al. (1988) Br. J. Haematol. 69:359.
The ED50 for this effect is 0.05-0.5 ng/mL.
The specific activity of Recombinant Human Thrombopoietin is >1 x 107 units/mg, which is calibrated against the human Thrombopoietin reference standard (NIBSC code: 03/124).
The ED50 for this effect is 0.05-0.5 ng/mL.
The specific activity of Recombinant Human Thrombopoietin is >1 x 107 units/mg, which is calibrated against the human Thrombopoietin reference standard (NIBSC code: 03/124).
Host Cell Protein
<5.00 ng per μg of protein when tested by ELISA.
Mycoplasma
Negative for Mycoplasma.
Host Cell DNA
< 0.0010 ng per µg of protein when tested by PCR.
Scientific Data Images for Recombinant Human Thrombopoietin GMP Protein, CF
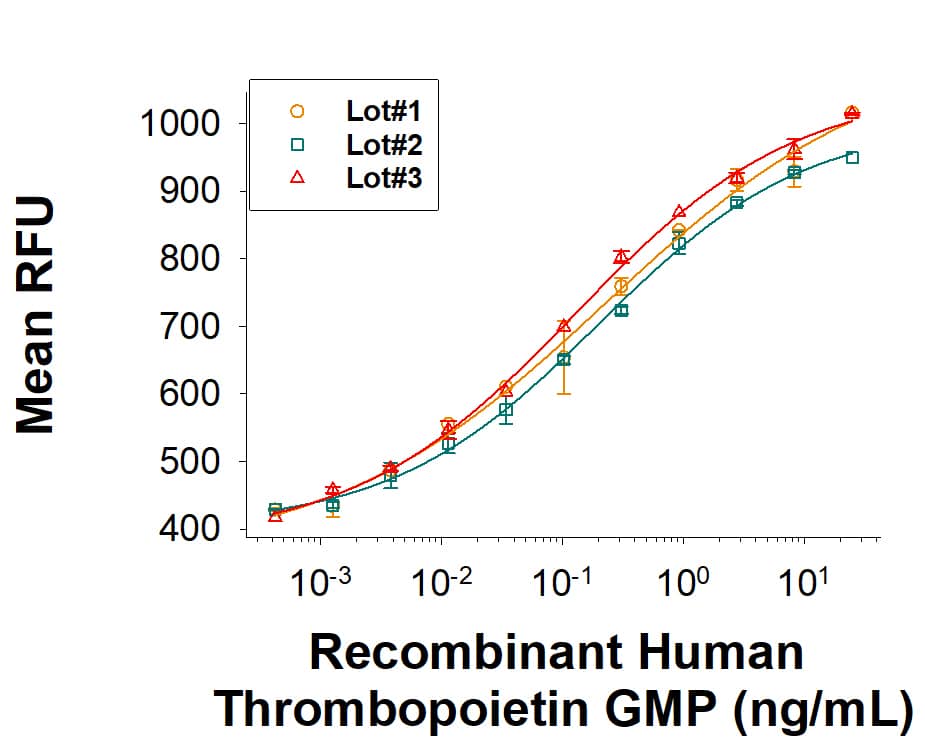
Recombinant Human Thrombopoietin GMP Protein Bioactivity
GMP-grade Recombinant Human Thrombopoietin (Catalog # 288E-GMP) stimulates proliferation in the MO7e human megakaryocytic leukemic cell line. The ED50 for this effect is 0.05-0.5 ng/mL. Three independent lots were tested for activity and plotted on the same graph to show lot-to-lot consistency of GMP Thrombopoietin.Recombinant Human Thrombopoietin GMP Protein SDS-PAGE
2 μg/lane of GMP-grade Recombinant Human Thrombopoietin (Catalog # 288E-GMP) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 19 kDa and 18 kDa, respectively.Formulation, Preparation and Storage
288E-GMP
| Formulation | Lyophilized from a 0.2 μm filtered solution in Sodium Acetate. |
| Reconstitution | Reconstitute at 100-200 μg/mL in sterile, deionized water. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: Thrombopoietin/Tpo
References
- Deutsch, V.R. and A. Tomer (2006) Br. J. Haematol. 134:453.
- Kaushansky, K. (2005) J. Clin. Invest. 115:3339.
- Li, J. et al. (1999) Br. J. Haematol. 106:345.
- Bartley, T.D. et al. (1994) Cell 77:1117.
- de Sauvage, F.J. et al. (1994) Nature 369:533.
- Marcucci, R. and M. Romano (2008) Biochim. Biophys. Acta 1782:427.
- Kaushansky, K. et al. (1994) Nature 369:568.
- Kaushansky, K. et al. (1995) Proc. Natl. Acad. Sci. 92:3234.
- Broudy, V.C. et al. (1995) Blood 85:1719.
- Lok, S.I. et al. (1994) Nature 369:565.
- Chen, J. et al. (1995) Blood 86:4054.
- Oda, A. et al. (1996) Blood 87:4664.
- Van Os, E. et al. (2003) Br. J. Haematol. 121:482.
- Kato, T. et al. (1997) Proc. Natl. Acad. Sci. 94:4669.
- Foster, D. & Hunt, P. (1997) Thrombopoiesis and Thrombopoietins 13:203.
- Ehrenreich, H. et al. (2005) Proc. Natl. Acad. Sci. 102:862.
- Samoylenko, A. et al. (2008) Cell. Signal. 20:154.
Alternate Names
MGDF, MK-CSF, MKCSF, MPLLG, THCYT1, THPO, Tpo
Gene Symbol
THPO
UniProt
Additional Thrombopoietin/Tpo Products
Product Documents for Recombinant Human Thrombopoietin GMP Protein, CF
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Designed, manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented and controlled manufacturing process
- Control of documentation and process changes by QA
- Personnel training programs
- Raw material inspection and vendor qualification/monitoring program
- Validated equipment, processes and test methods
- Equipment calibration and maintenance schedules using a Regulatory Asset Manager
- Facility/Utilities maintenance, contamination controls, safety and pest control programs
- Material review process for variances
- Robust product stability program following relevant ICH guidelines
- N-terminal amino acid analysis
- SDS-PAGE purity analysis
- Molecular weight analysis via mass spectrometry
- Endotoxin assessment per USP <85> and Ph. Eur. 2.6.14 guidelines
- Bioassay analysis
- Microbial testing per USP <71> and Ph. Eur. 2.6.1 guidelines
- Host cell protein assessment
- Host cell DNA assessment
- Mycoplasma assessment
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis and St. Paul, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Animal-Free Manufacturing Conditions
Our dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers.
Product Specific Notices for Recombinant Human Thrombopoietin GMP Protein, CF
Full terms and conditions of sale can be found online in the Protein Sciences Segment T&Cs at: Terms & Conditions.
For preclinical, or clinical ex vivo use
Loading...
Loading...
Loading...