| Key Benefits: |
Learn more about Fluorescent Glycan Labeling and Detection
Summary for GDP-Azido-Fucose
Incorporate or detect fucose without expensive, specialized equipment! Applications - For in vitro enzymatic incorporation of azido-sugars into specific, targeted glycans.
- Detecting the presence or absence of fucosylated glycans.
- Tagging bio-molecules through fucosylation.
- Assessing the level of fucosylation on target molecules.
Key Features and Benefits - Can assess for the presence of terminal fucosylation (e.g. core fucose) without expensive, specialized equipment.
- Can be introduced to proteins and lipids that have fucosylation sites via fucosyltransferases.
- Can be conjugated to desired reporter molecules via click chemistry.
- Can be detected via Western blot, ELISA, and flow cytometry, depending on the type of reporter molecule.
- Contains the smallest possible orthogonal functional group.
- Has minimal side effects on target molecules.
- User-friendly.
Related Reagents Click Chemistry Enzymes and Detection Reagents for GDP-Azido-Fucose, ES101 Schematic | 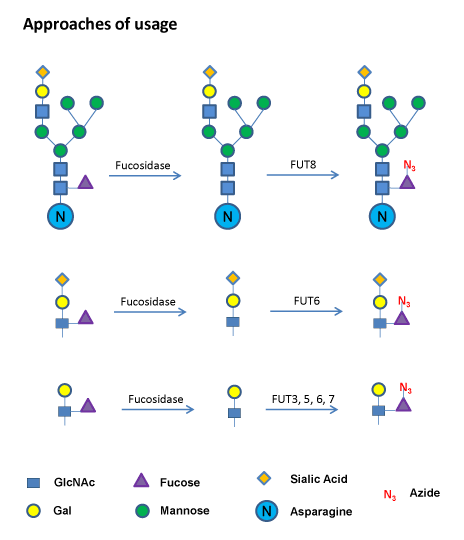
| The terminal fucose can be removed using a Fucosidase. Azido-fucose can then be added to open sites with specific fucosyltransferases and detected using Biotinylated Alkyne in a click chemistry reaction. | Sample Data | 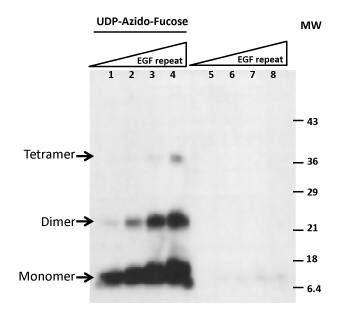
| Detecting Open Fucosylation Sites on the Acceptor Substrate Recombinant Drosophila Notch EGF20. rhPOFUT1 at 0.5 µg of was incubated at 37 °C for one hour with increasing amounts (0.5, 1, 2, 4 µg) of rdNotch EGF20 repeat. The left side is a labeling reaction including GDP-Azido-Fucose. The right side serves as a negative control labeling reaction without GDP-Azido-Fucose. The results clearly show GDP-Azido-Fucose incorporation into the Notch EGF20 substrate on open sites for POFUT1-mediated fucosylation. The buffer used contained 25 mM HEPES pH 7.5, 150 mM NaCl, and 10 mM MnCl2 for a total volume of 40 µl. The reactions were then conjugated for one hour with 1 nmol of Biotinylated Alkyne in the prescence of 100 µM CuCl2 and 2 mM Ascorbic Acid for a final volume of 50 µl. All reactions were separated on 12% SDS-PAGE and then detected with Streptavidin-HRP. |
Product Specifications for GDP-Azido-Fucose
| Species |
Multi-Species
|
Preparation & Storage
| Shipping Conditions |
The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below.
|
| Storage |
Store the unopened product at < -70 °C. Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Good for 12 months from date of receipt.
|
Assay Procedure
Sample Protocol for Core-6 Fucose Labeling Protocols are guidelines. Parameters need to be optimized by end users. Materials - Assay Buffer: 25 mM HEPES, 150 mM NaCl, 10 mM MnCl2, pH 7.5.
- Protein Sample
- Recombinant Human Fucosyltransferase 8/FUT8 (R&D Systems, Catalog # 5768-GT)
- GDP-Azido-Fucose (R&D Systems, Catalog # ES101)
- Biotinylated Alkyne (R&D Systems, Catalog # ES100)
- CuCl2, 1 mM in deionized water
- Ascorbic Acid, 20 mM in deionized water
- SDS-PAGE and Western blot reagents or equivalent
- TBST buffer: 25 mM Tris, 137 mM NaCl, 0.1% Tween-20, pH 7.5
- Streptavidin-HRP (R&D Systems, Catalog # DY998)
Assay Procedure - Prepare a reaction mixture by combining 5 µg of Protein Sample, 1 µg of rhFUT8 in the presence of 1 nmol GDP-Azido-Fucose in the Assay Buffer with the final volume of 25 µL.
- Prepare negative controls according to step 1 but omit Protein Sample or rhFUT8 or GDP-Azido-Fucose.
- Incubate all the reactions and the controls at 37°C for one hour.
- Add to each of the samples: 5 µL of 1 mM CuCl2, 5 µL of 20 mM Ascorbic Acid, and 5 µL of 1 mM Biotinylated Alkyne. Mix with gentle tapping.
- Incubate all samples at room temperature for 1 hour.
- Separate the reactions and controls by 12% SDS-PAGE.
- Blot the gel to a nitrocellulose membrane.
- Block the blot with 10% fat-free milk for 5 minutes.
- Thoroughly wash the membrane with TBST buffer by changing buffer three times for a total of 45 minutes.
- Incubate the blot with 25 ng/mL Streptavidin-HRP in 30 mL TBST buffer for 30 minutes.
- Thoroughly wash the membrane with TBST buffer by changing buffer three times for a total of 45 minutes.
- Detect with commercial ECL (Enhanced Chemiluminescence) reagents.
Final Assay Conditions Per Reaction - GDP-Azido-Fucose: 1 nmol
- rhFUT8: 1 µg
- Protein Sample: 5 µg
- Reaction volume: 25 µl
Click Chemistry Reaction Conditions Per Reaction - CuCl2: 5 nmol
- Ascorbic Acid: 100 nmol
- Biotinylated Alkyne: 5 nmol
- Reaction volume: 40 µl
Customer Reviews for GDP-Azido-Fucose
Have you used GDP-Azido-Fucose?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Product Documents for GDP-Azido-Fucose
|





