Measure Biodistribution, Cellular Tropism and Transduction Efficiency
RNAscope™ in situ hybridization (ISH) assays may be used in gene therapy to provide spatial biodistribution analysis of viral AAV vector, transgene mRNA and non-viral oligonucleotide therapeutics (ASO/siRNA) with single cell resolution. RNAscope probes can be designed to target unique regions within the promoter, regulatory elements, the transgene of a viral vector or short oligonucleotide sequences used in RNAi therapies.
Why Choose RNAscope Technology for Gene Therapy?
- Visualize tissue biodistribution and cellular tropism of viral vectors such as AAV, transgene and RNA therapeutics delivered by non-viral platforms with single-molecule sensitivity and sub-cellular resolution.
- Select engineered vectors and capsids for highest transduction efficiency.
- Quantify expression and persistence of transgenes including codon-optimized or gene-edited cargo.
- Evaluate therapeutic efficacy including RNA efficiency within targeted cells and tissues.
- Improve safety by evaluating dosing and identifying off-target events in relevant organs.
Unlock Answers to Critical Questions
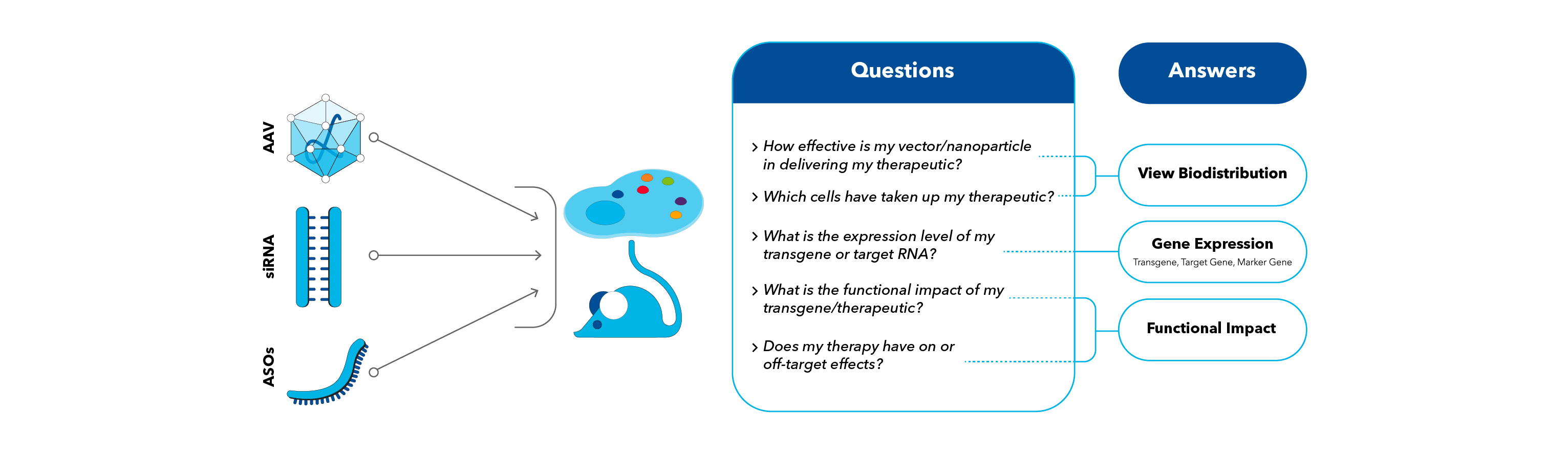
Visualize Biodistribution
Visualize Biodistribution
- Optimize the targeting efficiency of your therapeutic using any preclinical animal model.
- Easily measure biodistribution, cellular tropism and transduction efficiency across multiple tissues of interest with high specificity.
Single Cell Gene Expression
Single Cell Gene Expression
- Evaluate the performance of your engineered cargo in vivo.
- Quantify the expression and persistence of your transgene or therapeutic target with single-molecule sensitivity.
Functional Impact
Functional Impact
- Demonstrate the functional efficacy and safety in disease models.
- Visualize changes in targeted cells and tissues in spatial context using both protein and RNA biomarkers.
- Monitor off target events by visualizing biodistribution of the therapy in various organs.
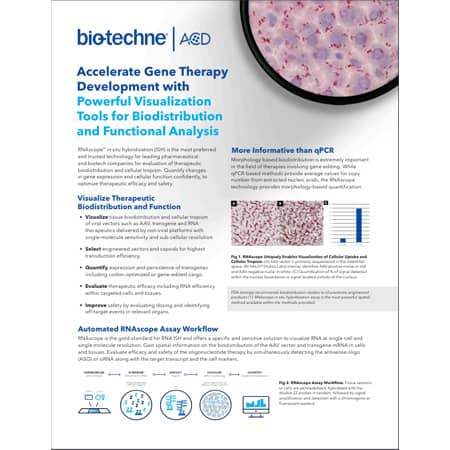
More Informative than qPCR
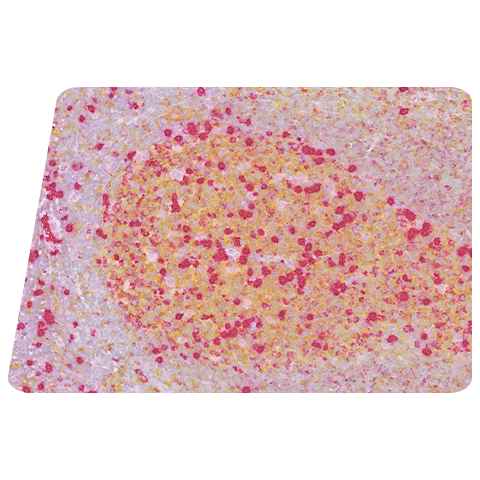
Evaluate Subcellular Localization of Vector Delivery and Transgene Expression: Morphology-based biodistribution is extremely important where a therapy involves gene editing. While qPCR-based methods provide average values for copy number from extracted nucleic acids, the RNAscope technology provides morphology-based quantification.
Figure: RNAscope uniquely enables visualization of cellular uptake and cellular tropism. (A) AAV vector is primarily sequestered in the interstitial space. (B) HALO ® (Indica Labs) overlay identifies AAV-positive nuclei in red and AAV-negative nuclei in white. (C) Quantification of % of signal detected within the nuclear boundaries vs signal located outside of the nucleus.


RNAscope Probes Redefine Biodistribution Monitoring with Unmatched Specificity
RNAscope is a trusted technology used in >12,000 publications with multiple ordering formats to choose from. We offer ultimate flexibility in RNAscope probe design and can easily design probes specific to your proprietary therapeutic, for use in any animal model system. Our probes enable precise distinction between human transgenes and cross-species orthologs in animal models and allow accurate differentiation between codon-optimized transgenes and native sequences.
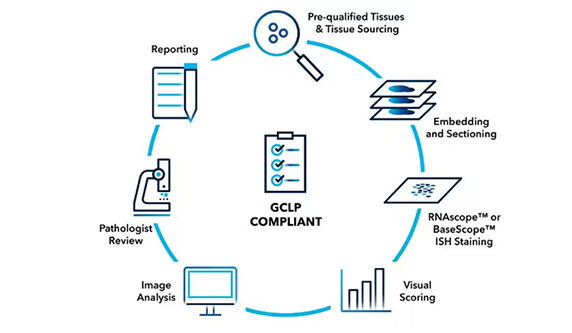
RNAscope Professional Assay Services
Our Professional Assay Services offer you direct access to the developers of RNAscope in situ hybridization (ISH) technology. Accelerate your journey in target validation, preclinical, and clinical development with the promise that we will provide consistent and trustworthy data to drive your projects forward.
RNAscope ISH Technology - Product Formats
Multiomic Spatial Solutions for RNA and Protein Co-detection
RNAscope~20 ZZ probe pairs Designed to > 300 nt.
| BaseScope™1-3 ZZ probe pairs Designed to ~ 50-300 nt.
| miRNAscope™Designed to ~17-50 nt.
| RNAscope PlusRNA species of varied sizes.
|
Reference
- S12 Nonclinical Biodistribution Considerations for Gene Therapy Products; Guidance for Industry; U.S. Department of Health and Human Services Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER) May 2023; ICH-Safety.