By Christina Towers, PhD
Is p62 a good indicator of autophagic flux? The short answer: Yes … but … SQSTM1 encodes the cargo adaptor protein, p62, which interacts with autophagic substrates and delivers them to autophagosomes for degradation. In the process, p62 is itself degraded and when autophagy is induced, a corresponding decrease in p62 levels is observed. Congruently, when autophagy is potently inhibited, i.e. genetic knock out of ATG core autophagy proteins like ATG5 or ATG7, p62 accumulates in the cell. These changes can be observed by immunofluorescent staining, western blotting, and immunohistochemistry in the case of intact tissue samples.
|
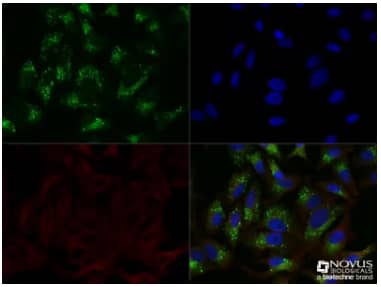
Biological Strategies Validation. Immunocytochemistry/Immunofluorescence: p62/SQSTM1 Antibody [NBP1-48320] - HeLa cells were treated overnight with 50uM CQ, then fixed for 10 minutes using 10% formalin and then permeabilized for 5 minutes using 1X TBS + 0.5% Triton X-100. The cells were incubated with anti-p628/SQSTM1 at a 1:200 dilution overnight at 4C and detected with an anti-rabbit Dylight 488 (Green) at a 1:500 dilution. Alpha tubulin (DM1A)NB100-690 was used as a co-stain at a 1:1000 dilution and detected with an anti-mouse DyLight 550 (Red) at a 1:500 dilution. Nuclei were counterstained with DAPI (Blue). Cells were imaged using a 40X objective. |
p62 is a versatile adaptor protein with multiple functions
Interestingly, p62 itself is thought to be dispensable for canonical autophagy. In contrast to almost all of the core ATG proteins whose loss in mice results in embryonic or neonatal lethality, the only adverse phenotype displayed in p62-/- mice is mature-onset obesity1. A number of recent publications have shown that p62 is a multifunctional scaffolding protein that interacts with a variety of proteins to regulate diverse processes including apoptosis and other forms of cell death like necroptosis, as well as redox state via regulation of the KEAP1-NRF2 pathway. p62 has also been shown to interact with ERK1 to regulate adipogenesis and protein kinase C (PKC zeta) to regulate NF-κB signaling2.
Always consider alternative assays to monitor autophagic flux
These diverse roles suggest that homeostatic regulation may affect p62 levels independent of autophagy. For this reason, it is important to validate changes in autophagic flux as measured by p62 levels, with additional assays. Most commonly, conjugated levels of LC3, known as LC3-II, can be monitored by western blot in the presence of a lysosomal inhibitor such as bafilomycin-A1. Lysosomal delivery of specific autophagic cargo can also be monitored with the use of tandem tagged proteins that contain a pH stable mCherry fluorophore and a pH sensitive GFP fluorophore.
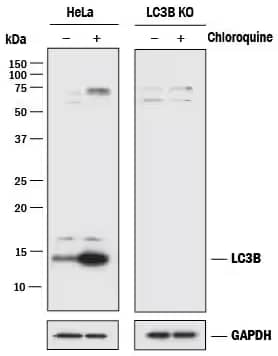
Genetic Strategies Validation. Knockout Validated: LC3B Antibody [NB100-2220] - Lysates of HeLa parental cell line and LC3B knockout HeLa cell line (KO) untreated (-) or treated (+) with 50 uM Chloroquine for 18 hours. PVDF membrane was probed with 0.5 ug/mL of Rabbit Anti-LC3B Polyclonal Antibody (Catalog # NB100-2220) followed by HRP-conjugated Anti-Rabbit IgG Secondary Antibody (Catalog# HAF008). A specific band was detected for LC3B at approximately 15 kDa (as indicated) in the parental HeLa cell line but is not detectable in the knockout HeLa cell line. GAPDH is shown as a loading control. This experiment was conducted under reducing conditions.
An advantage to monitoring p62 to measure autophagic flux is that lysosomal inhibitors are not necessary, because unlike LC3-II, p62 does not usually increase when autophagy is induced. However, changes in p62 can often be subtle compared to LC3-II flux, probably because of additional mechanisms of regulation. For these reasons, it is important to always use multiple assays to measure autophagy flux3.

Christina Towers, PhD
University of Colorado (AMC)
Dr. Towers studies the roles of autophagy, apoptosis and cell death in cancer.
-
Kuma, A. et al. (2017) Autophagy-monitoring and autophagy-deficient mice Autophagy
-
Sánchez-Martín, P. et al. (2018) p62/SQSTM1 – steering the cell through health and disease Journal of Cell Science
-
Klionsky, D. J. et al. (2012) Guidelines for the use and interpretation of assays for monitoring autophagy Autophagy