What is Autophagy?
Autophagy plays critical roles during organismal development and immune response, by regulating growth and cooperating with the adaptive immune system. In disease states, such as neurodegenerative conditions, inefficient autophagic activity leads to the accumulation of abnormal proteins and formation of intracellular aggregates. Autophagy provides a mechanism to battle and eliminate infectious pathogens. In cancer, autophagy plays a role in promoting or inhibiting tumor growth in a system and stage dependent manner.
Featured Autophagy Products
Rapid Autophagy Biomarker Discovery
Rapid Autophagy Biomarker Discovery
Unlock the future of Autophagy Research with Bio-Techne's Simple WesternTM Antibodies. Achieve precision, versatile protein separation, and a multiplexed platform for effortless biomarker discovery.
Maximize Your Antibody Value
Maximize Your Antibody Value
Streamline your search for Autophagy research antibodies with Bio-Techne’s carefully curated sampler packs, offering multiple antibodies targeting autophagy-related proteins.
Simplified Quantification for Autophagy Research
Simplified Quantification for Autophagy Research
Effortlessly find the perfect ELISA kit for your autophagy research from a variety of formats, including indirect, sandwich, and competitive ELISAs
Autophagy Inducers and Inhibitors
Small molecules able to inhibit or induce autophagic activity provide a valuable mechanism to modulate and study autophagy. Autophagy can be induced both in vivo and in vitro, enabling autophagy research at the single-cell level up to whole-organism scale. One of the most reliable methods to induce autophagy is the use of autophagy-inducing peptides Tat-Beclin 1 D11 (Tat-D11) and Tat-Beclin 1 L11 (Tat-L11). Discover more about these high-specificity peptides on our Autophagy-Inducing Peptides page.
Related Categories
Types of Autophagy
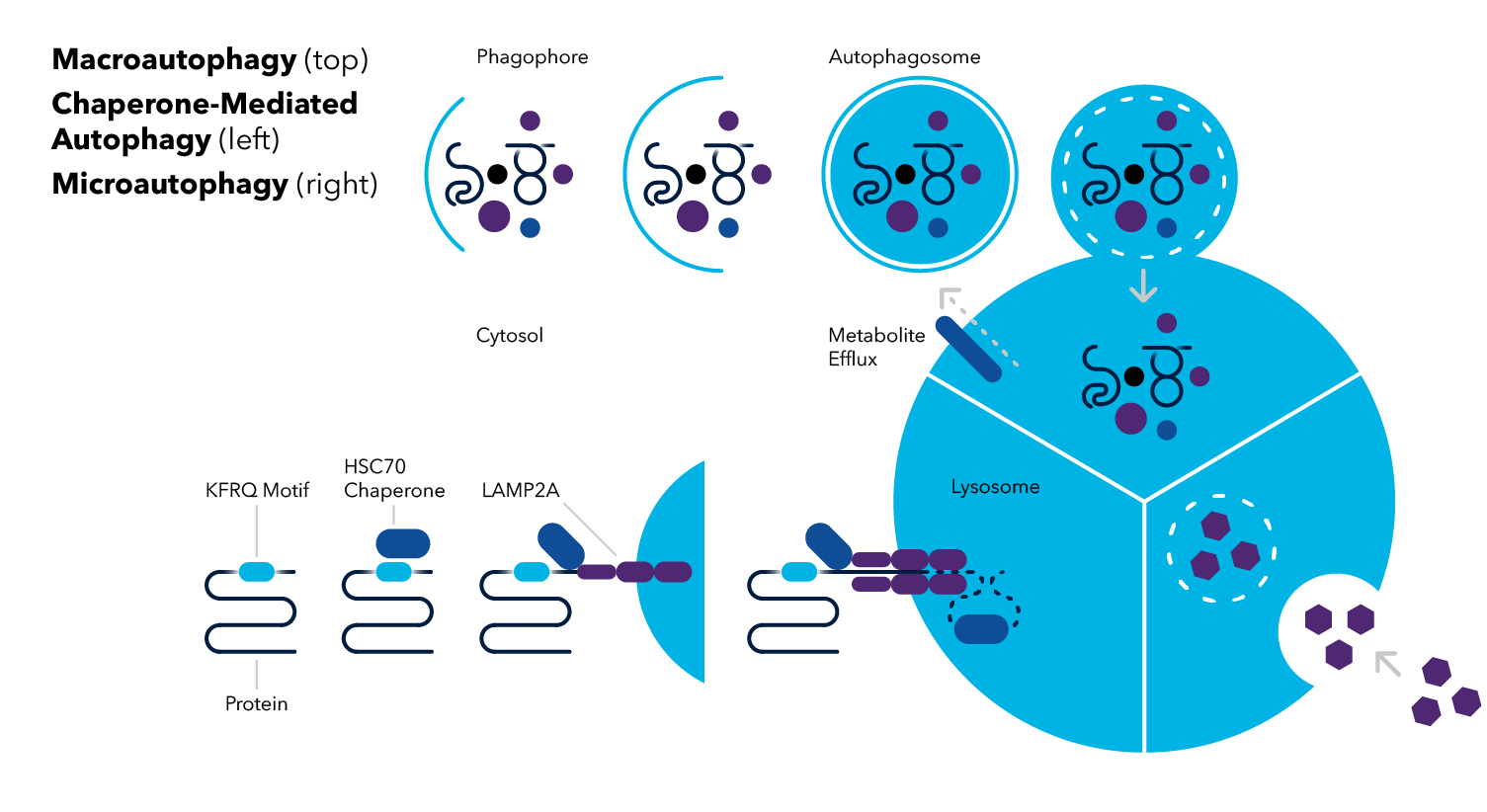
Macroautophagy
Macroautophagy involves the sequestering of cytosolic components within a double membrane organelle called the autophagosome, transport to the lysosome, and the subsequent cargo degradation. Hereafter, macroautophagy is referred to as autophagy.
Autophagy can occur either via non-selective (bulk) degradation or through selective elimination of cytosolic components including pathogens, protein aggregates, and dysfunctional organelles. Many organelles have specific autophagy pathways for functional maintenance such as mitophagy (mitochondria), ER-phagy (endoplasmic reticulum), pexophagy (peroxisomes), and lysophagy (lysosomes).
The process of autophagy is divided into a series of steps: induction, nucleation and phagophore formation, elongation and autophagosome formation, fusion with the lysosome to form the autolysosome, and degradation. In the initial step, the dephosphorylation of ULK1 increases the activity of the ULK1/ATG13/ATG101 complex, and induces autophagy. Autophagy-related (ATG) proteins and complexes are at the center of many of these steps and multiple ATG proteins are critical during elongation and autophagosome formation. Briefly, ATG7 mediates ATG5-ATG12-ATG16L1 complex formation and together with ATG4 and ATG3 processes pro-LC3 to phosphatidylethanolamine (PE) lipid-conjugated form, LC3-II, which associates with the autophagosome membrane.
Microautophagy
Microautophagy is the process where lysosomes directly engulf cytosolic components via lysosomal membrane invagination or protrusion without prior formation of an autophagosome. The vacuole containing cargo separates from the membrane, becoming internalized within the lysosome as a microautophagic body. Similar to what is observed in macroautophagy, the microautophagic body is lysed and the contents are broken down by vacuolar hydrolases into macromolecules that can be recycled.
Chaperone-Mediated Autophagy
Chaperone-mediated autophagy (CMA) is unique from the other two types of autophagy in that the process does not involve generation of autophagic bodies. Similar to microautophagy, CMA also occurs independently of the autophagosome, however, CMA does not involve lysosomal invagination. Instead, chaperone proteins (e.g., heat shock cognate 70 protein; HSC70) recognize cytosolic cargo destined by degradation by their consensus sequence known as the KFERQ-like motif. This chaperone-cargo complex associates with membrane-bound lysosomal-associated membrane protein-2A (LAMP-2A), resulting in the translocation of the unfolded cytosolic protein into the lysosome.
Want to learn more about the key signaling pathways involved in the different types of autophagy? Our autophagy signaling pathway page has a host of useful resources to help you.
Autophagy Detection Methods
Primary Antibodies by Application
| ELISA | Flow Cytometry | Immunocytochemistry/ Immunofluorescence (ICC/IF) |
| Immunohistochemistry (IHC) | Simple Western | Western Blot |
Traditionally, the process of autophagy has been studied using electron microscopy. The discovery and characterization of ATG proteins facilitated the development of molecular tools and approaches to identify and quantitate autophagic activity. Ideally, a combination of approaches should be implemented including steady-state and flux measurements for the assessment of autophagic activity in different systems.
Steady-State Assay: Autophagosome Number
Assays to determine the amount or number of autophagosomes generally focus on the LC3 protein. LC3 may be found in the cytosolic and nuclear compartments. The lipidated form or LC3-II is the only protein known to associate with the autophagosome’s inner membrane. LC3-II levels, detected by immunofluorescence (ICC/IHC/IF) and immunoassay (WB) provide a good estimation of autophagosome number.
Monitoring Autophagic Flux
Autophagic flux refers to the complete processing of cargo, from sequestration to its degradation and recycling of basic components back to the cytosol. Increased LC3-II signal in immunofluorescence or immunoblot assays may result from increased autophagy. However, increased LC3-II or autophagosome number may also result from a blockade in autophagic flux. Therefore, to clearly distinguish between these mechanisms, assays that measure autophagic flux should be combined with steady-state assays.
|
Assay |
Method |
Activity |
Protocols and Resources |
|
LC3-II Puncta |
ICC/IHC/IF |
Autophagosome Number |
Inhibition of Autophagy and LC3B Antibody (NB100-2220) Immunocytochemistry (ICC) Immunohistochemistry-Paraffin Protocol for LC3B/MAP1LC3B Antibody (NB100-2220) |
|
LC3 Conversion |
WB |
Autophagosome Number |
LC3/MAP1LC3A Antibody Pack [NB910-40752] |
|
LC3 Turnover Assay |
WB, ICC/IF |
Autophagic flux |
Inhibition of Autophagy and LC3B Antibody (NB100-2220) Western Blot Inhibition of Autophagy and LC3B Antibody (NB100-2220) Immunocytochemistry (ICC) |
|
p62/SQSTM1 Degradation Assay |
WB, Flow, ELISA, ICC/IF |
Autophagic flux |
|
|
WB, IP, Kinase Assay |
Autophagosome Number/ Autophagic flux |
||
|
ICC/IF |
Autophagosome Number/ Autophagic flux |
Resources
Featured Resource: Autophagy Handbook
Discover valuable insights into targets and tools for accurately quantifying autophagic activity and flux, empowering you to track and analyze autophagy with precision.