Jamshed Arslan, Pharm D, PhD
SARS-CoV-2 induces both humoral and cellular immunity. A vaccine or natural infection invokes SARS-CoV-2-specific humoral components (antibodies from activated B cells) and cellular response (CD4+, CD8+ T cells) that can protect against subsequent infections. Following SARS-CoV-2 infection, the levels of viral spike (S) protein-specific antibodies and virus-specific memory T cells decrease over time, but the correlation between viral load, immunological parameters, and disease severity and outcome is not straightforward. To explore this interrelationship, a team in Singapore studied virus-specific responses in symptomatic COVID-19 patients for a month, followed by additional sampling afterwards. The researchers concluded that it is primarily the early virus-specific T cell immunity, not the humoral response, that leads to a rapid viral control.
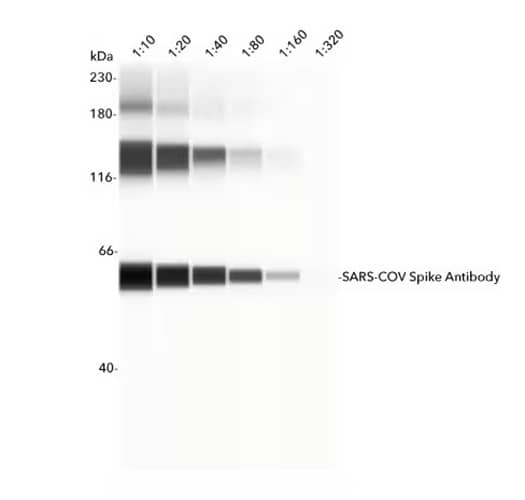
Simple Western lane view showing recombinant SARS-CoV-2 Spike S2 Protein detected using Rabbit Anti-SARS-CoV Spike Protein Polyclonal Antibody (NB100-56578) in serial dilutions from 1:10 – 1:200. A specific band for SARS-CoV-2 Spike S2 Protein is identified at 58 kDa (as indicated). Primary antibody probing was followed by incubation with HRP-conjugated Secondary Antibody. This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
Antibodies and cellular response to SARS-CoV-2
To study the dynamics of immune response, the team estimated viral quantity in the respiratory tract of 12 symptomatic patients using RT-PCR cycle as a surrogate measure. They found that the time between symptom onset and SARS-CoV-2 RT-PCR negativity was associated with high viral quantity. Analysis of patients’ serum antibodies (IgG and IgM) showed peak neutralization of viral S1, receptor binding domain (RBD), and nucleoprotein (NP) in the second week after symptom onset. The antibody response was stronger in patients with moderate/severe symptoms compared to the mild cases.
To explore the cellular response, the researchers stimulated patients’ peripheral blood mononuclear cells (PBMCs) with viral antigens in an IFN-γ Enzyme-Linked Immunospot (ELISpot) assay. Similar to the humoral response, the IFN-γ-secreting cells increased following stimulation, with peak detected in the second week after symptom onset. However, unlike the antibody response, IFN- γ-secreting cells were more frequent in early and late stages of the milder cases as compared to the moderate/severe cases. Most importantly, the shorter duration of infection was directly correlated with early cellular response, such as SARS-CoV-2 peptide-reactive cells specific for ORF7/8, ORF3a, NP, membrane (M), and S protein, but not with the antibody response.
After establishing a link between the appearance of functional T cells with shorter duration of infection, the team analyzed the ability of viral proteins to elicit a cellular response (IFN-γ-production) in both mild and severe cases.
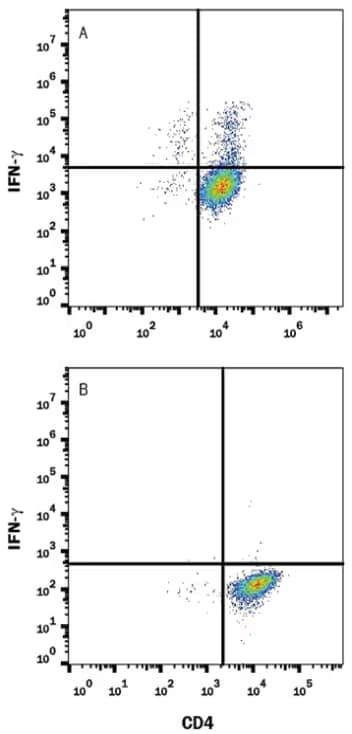
Mouse splenocytes stimulated (A) of unstimulated (B) to induce Th1 cells and stained with Anti-Mouse IFN‑ gamma Monoclonal Antibody (MAB485) and then followed by staining with PE-conjugated IgG Secondary Antibody (F0105B) and Anti-Mouse CD4 APC‑conjugated Monoclonal Antibody (FAB554A). IgG2a Isotype Control Antibody (MAB006) staining was used to set quadrant markers. Cells were fixed with Flow Cytometry Fixation Buffer (FC004-NOV) and permeabilized with Flow Cytometry Permeabilization/Wash Buffer I (FC005).
T cell response in mild and severe COVID-19
The researchers found that among the peptide pools covering SARS-CoV-2 proteins, ORF7/8 accessory protein distinctly initiated a robust IFN-γ response in the early phase of mild, but not severe, infection. Such response completely waned after a month of viral clearance. The phenotypic analysis of IFN-γ-producing cells through flow cytometry revealed a vast proportion of CD4+ T cells in response to viral proteins, with some NP-specific CD8+ T cells.
These result shows that the viral proteins, especially ORF7/8, trigger a strong T cell response in the early phase of SARS-CoV-2 infection, which serves to clear the virus.
Future directions
This longitudinal research experiment used only 12 patients and relied only on ELISpot for detecting IFN-γ-producing cells to show the prognostic value of T cells in COVID-19 patients. Similar longitudinal studies are warranted, but with greater sample size and multiple functional assays, to determine the effects of age, sex, ethnicity, and other additional factors on immune response to SARS-CoV-2.
Note:
Tan et al. (2021) used Anti-Human IFN-γ PE (Catalog # IC285P) and Recombinant Human IL-2 protein (Catalog # 202-1L-050).

Jamshed Arslan, Pharm D, PhD
Dr Arslan is an Assistant Professor at Salim Habib University (formerly, Barrett Hodgson University), Pakistan. His interest lies in neuropharmacology and preparing future pharmacists.