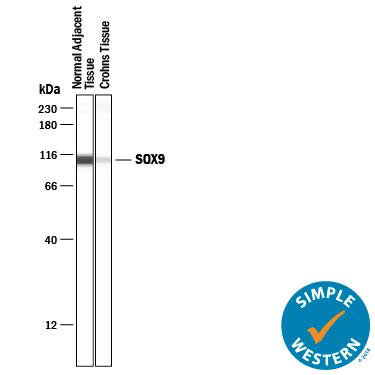
Detection of Human SOX9 by Simple WesternTM.
Simple Western lane view shows lysates of normal adjacent tissue and Crohns tissue, loaded at 0.2 mg/mL. A specific band was detected for SOX9 at approximately 107 kDa (as indicated) using 20 µg/mL of Goat Anti-Human SOX9 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3075) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (
HAF019). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
Detection of Mouse SOX9 by Western Blot
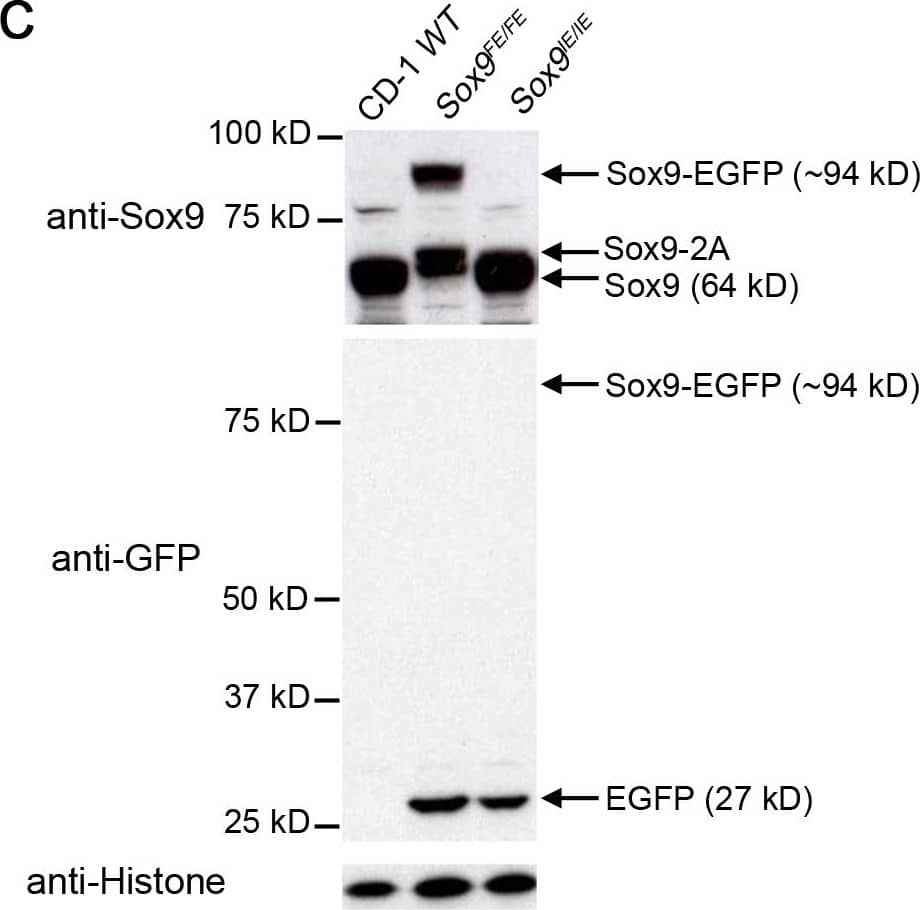
Comparison of E12.5 Sox9IE/IE and Sox9FE/FE embryos based on EGFP intensity and Western blotting.(A) Left panel: white light images of E12.5 Sox9IE/IE and Sox9FE/FE embryos; right panel: same embryos imaged by fluorescence microscopy showed a higher EGFP fluorescence in the Sox9FE/FE embryo compared to the Sox9IE/IE embryo, with EGFP expression in Sox9-specific domains in both. (B) Mean overall EGFP fluorescence of E12.5 Sox9IE/IE (13880.04±1015.55; n = 8) and Sox9FE/FE (84579.48±2822.09; n = 4) embryos analyzed by flow cytometry. Overall EGFP fluorescence was calculated by multiplying the MFI and percentage of EGFP-positive cells. Two-tailed Student's T test, ***p<0.0001. (C) Western blot analysis of E12.5 Sox9IE/IE and Sox9FE/FE embryo lysates, resolved on SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gels and immunoblotted with anti-Sox9 (upper panel) and anti-GFP (middle panel) antibodies. In the upper panel, Sox9-EGFP fusion protein and Sox9-2A (2A - residual 23 amino acids of F2A) was only detected in the Sox9FE/FE embryo lysate, but not in the CD-1 WT or Sox9IE/IE embryo lysates. Middle panel: Sox9-EGFP fusion protein was not detected in the Sox9FE/FE embryos but EGFP was detected in both the Sox9IE/IE and Sox9FE/FE lysates at predicted molecular weight. CD-1 WT lysate served as negative control for EGFP. Bottom panel: immunoblotting with anti-histone antibody showed equal loading. WT – wild-type. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0028885), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse SOX9 by Immunocytochemistry/Immunofluorescence
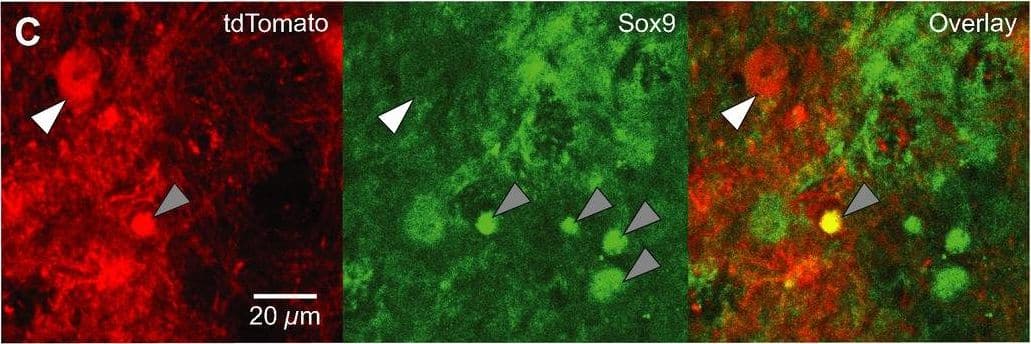
Dbx1‐expressing progenitors give rise to preBötC neurons and glia. A. Confocal images of Dbx1CreERT2; Rosa26tdTomato mouse preBötC sections immunostained for NeuN. Dbx1‐derived neurons (white arrowhead) expressed tdTomato and were immunoreactive for NeuN. Dbx1‐derived glia (gray arrowhead) expressed tdTomato and were not immunoreactive for NeuN. B. Confocal images of Dbx1CreERT2; Rosa26tdTomato mouse preBötC sections 72 h after injection with AAV‐hSyn‐GFP. Dbx1‐derived non‐neuronal cells extend diffuse fibrils that are closely apposed to the outer surface of microvasculature (white arrows). C, Confocal images of Dbx1CreERT2; Rosa26tdTomato mouse preBötC sections immunostained for Sox9. Glia (gray arrowheads) were immunoreactive for Sox9. Dbx1‐derived neurons (white arrowhead) expressed tdTomato but were not immunoreactive for Sox9. Dbx1‐derived glia expressed tdTomato and were immunoreactive for Sox9 (see “overlay” panel). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28611151), licensed under a CC-BY license. Not internally tested by R&D Systems.
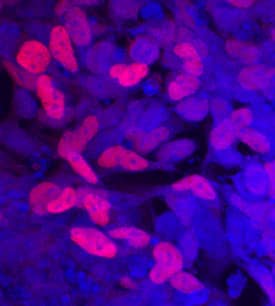
Detection of Human SOX9 by Immunocytochemistry/Immunofluorescence
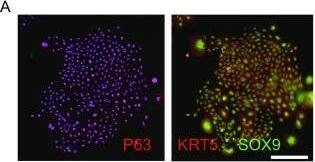
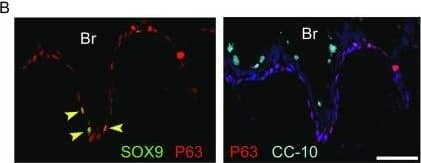
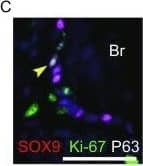
Feeder-free expansion of SOX9+ BCs. (A) Immunostaining of SOX9+ BCs with anti-P63, anti-KRT5 and anti-SOX9 antibodies. (B) SOX9+ BCs in rugae of 3rd order human airway by anti-SOX9, anti-P63 and anti-CC10 immunostaining. Scale bar, 100 μm. (C) SOX9+ BCs in rugae of 3rd order human airway by anti-KI67 immunostaining. (D) BC colony cultured on feeder-free condition. (E) Karyotyping of cultured BCs. (F) qPCR showing alveolar and bronchial epithelium marker gene expression of human lung sample and SOX9+ BCs in early (P2) and late (P8) passages. n = 3, biological replicates. Error bars, S.E.M. (G) qPCR showing progenitor cell marker (Krt5, P63 and SOX9) gene expression of human lung sample and SOX9+ BCs in early (P2) and late (P8) passages. n = 3, biological replicates. Error bars, S.E.M. (H) Western blotting showing marker gene expression of human lung sample and SOX9+ BCs in early (P2) and late (P8) passages Image collected and cropped by CiteAb from the following publication (https://academic.oup.com/proteincell/article/9/3/267/6760074), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human SOX9 by Immunocytochemistry/Immunofluorescence
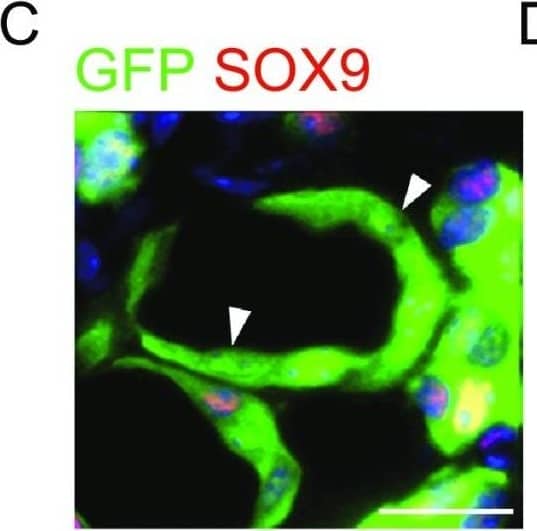
Transplantated SOX9+ BCs regenerate functional human lungin vivo. (A) Left, direct fluorescence image under stereomicroscope showing NOD-SCID mouse lung without (upper panel) or with (lower panel) GFP-labeled SOX9+ BC transplantation. Right, cryo-section and direct fluorescence imaging of transplanted GFP-labeled SOX9+ BCs in lung parenchyma. Scale bar, 100 μm. (B) Immunofluorescence imaging of transplanted GFP-labeled SOX9+ BCs in lung parenchyma with human specific Lamin A+C marker costaining. (C) Fully differentiated GFP+ cells lost SOX9 marker expression (arrowhead indicated). Scale bar, 10 μm. (D) Confocal image with human specific Lamin A+C immunostaining (HuLamin) showing regenerated type I (AQP5+) alveolar cells. No type II (SPC+) cells were observed. (E) Confocal image showing regenerated AEC1 (AQP5+ and HOPX+). AQP5 as a membrane-bound protein distributes on surface of GFP+ cells. Arrowheads indicated the overlay of HOPX with GFP signal in nucleus. Scale bar, 20 μm. (F) qPCR with human specific primers showing alveolar and bronchiolar epithelium marker gene expression in SOX9+ BC transplanted chimeric lung (AEC1: AQP5 and HOPX; AEC2: SPB and LAMP3; bronchiolar cells: SCGB1A1 and MUC1). Biological replicates, n = 3. Error bars, S.E.M. (G) Left, clonogenic BCs isolated from human cervix epithelium obtained by biopsy. Right, transplantation of equal numbers of BCs from lung and cervix indicated different incorporation efficiency Image collected and cropped by CiteAb from the following publication (https://academic.oup.com/proteincell/article/9/3/267/6760074), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human SOX9 by Immunocytochemistry/Immunofluorescence
Feeder-free expansion of SOX9+ BCs. (A) Immunostaining of SOX9+ BCs with anti-P63, anti-KRT5 and anti-SOX9 antibodies. (B) SOX9+ BCs in rugae of 3rd order human airway by anti-SOX9, anti-P63 and anti-CC10 immunostaining. Scale bar, 100 μm. (C) SOX9+ BCs in rugae of 3rd order human airway by anti-KI67 immunostaining. (D) BC colony cultured on feeder-free condition. (E) Karyotyping of cultured BCs. (F) qPCR showing alveolar and bronchial epithelium marker gene expression of human lung sample and SOX9+ BCs in early (P2) and late (P8) passages. n = 3, biological replicates. Error bars, S.E.M. (G) qPCR showing progenitor cell marker (Krt5, P63 and SOX9) gene expression of human lung sample and SOX9+ BCs in early (P2) and late (P8) passages. n = 3, biological replicates. Error bars, S.E.M. (H) Western blotting showing marker gene expression of human lung sample and SOX9+ BCs in early (P2) and late (P8) passages Image collected and cropped by CiteAb from the following publication (https://academic.oup.com/proteincell/article/9/3/267/6760074), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human SOX9 by Immunocytochemistry/Immunofluorescence
Feeder-free expansion of SOX9+ BCs. (A) Immunostaining of SOX9+ BCs with anti-P63, anti-KRT5 and anti-SOX9 antibodies. (B) SOX9+ BCs in rugae of 3rd order human airway by anti-SOX9, anti-P63 and anti-CC10 immunostaining. Scale bar, 100 μm. (C) SOX9+ BCs in rugae of 3rd order human airway by anti-KI67 immunostaining. (D) BC colony cultured on feeder-free condition. (E) Karyotyping of cultured BCs. (F) qPCR showing alveolar and bronchial epithelium marker gene expression of human lung sample and SOX9+ BCs in early (P2) and late (P8) passages. n = 3, biological replicates. Error bars, S.E.M. (G) qPCR showing progenitor cell marker (Krt5, P63 and SOX9) gene expression of human lung sample and SOX9+ BCs in early (P2) and late (P8) passages. n = 3, biological replicates. Error bars, S.E.M. (H) Western blotting showing marker gene expression of human lung sample and SOX9+ BCs in early (P2) and late (P8) passages Image collected and cropped by CiteAb from the following publication (https://academic.oup.com/proteincell/article/9/3/267/6760074), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Human SOX9 Antibody by Immunohistochemistry
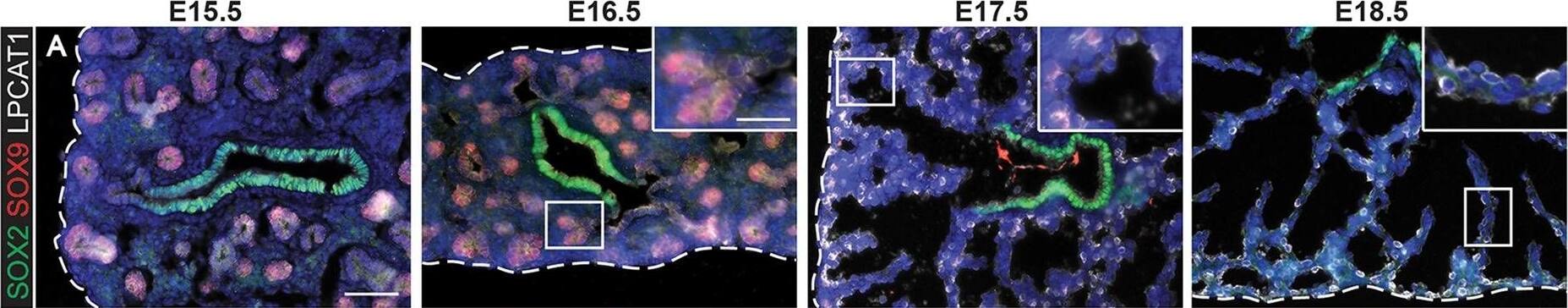
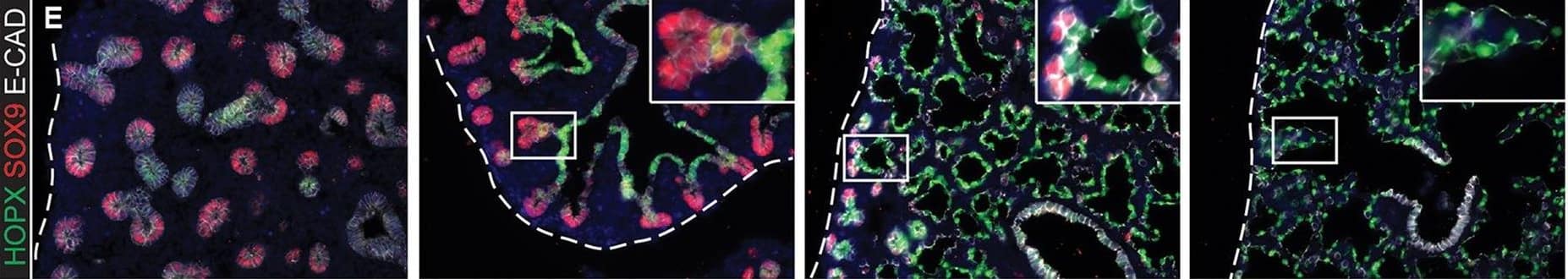
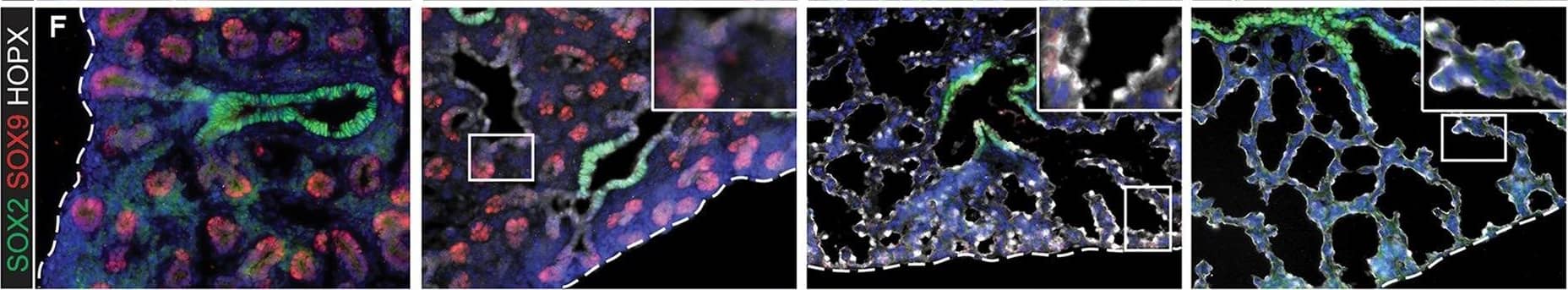
Evolution of alveolar epithelial gene expression patterns in the developing mouse lung. Sections of E15.5, 16.5, 17.5 and 18.5 wild-type mouse lungs stained for markers of differentiation. (A) Green, SOX2 (differentiating bronchioles); red, SOX9 (tips); white, LPCAT1 (tip cells from E16.5, then AT2 cells). (B) Green, CEBPA (sub-set of tip cells from E16.5, then AT2 cells); red, pro-SFTPC (embryonic epithelium, stronger from E16.5, later specific to AT2 cells). (C) Green, pro-SFTPC (stronger from E16.5, later specific to AT2 cells); red, LAMP3 (rare tip cells; AT2 cells); magenta, PDPN (tip cells from E16.5, then AT1 cells). (D) Green, LPCAT1 (tip cells from E16.5, then AT2 cells); red, LAMP3 (rare tip cells; AT2 cells); magenta, PDPN (tip cells from E16.5, then AT1 cells). (E) Green, HOPX (stalk cells from E16.5, AT1 cells); red, SOX9 (tip cells); white, E-CAD (epithelial cells). (F) Green, SOX2 (differentiating bronchioles); red, SOX9 (tips); white, HOPX (stalk cells from E16.5, AT1 cells). (G) Green, HOPX (stalk cells from E16.5, AT1 cells); red, LPCAT1 (tip cells from E16.5, then AT2 cells). Arrows, LPCAT1+ HOPX+ cells; arrowheads, LPCAT1+ HOPX− cells. Blue, DAPI (nuclei). Dashed line, edge of lung. Scale bars: 50 μm in A-F, 20 μm in G and insets. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27578791), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Human SOX9 Antibody by Immunohistochemistry
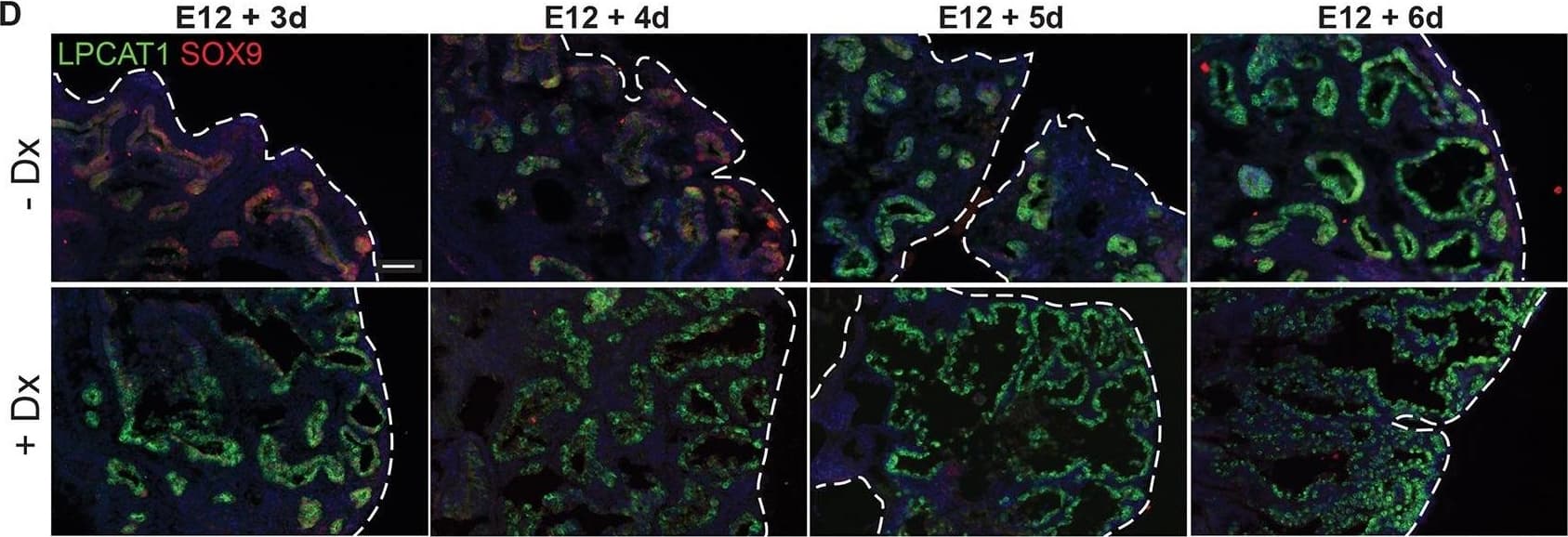
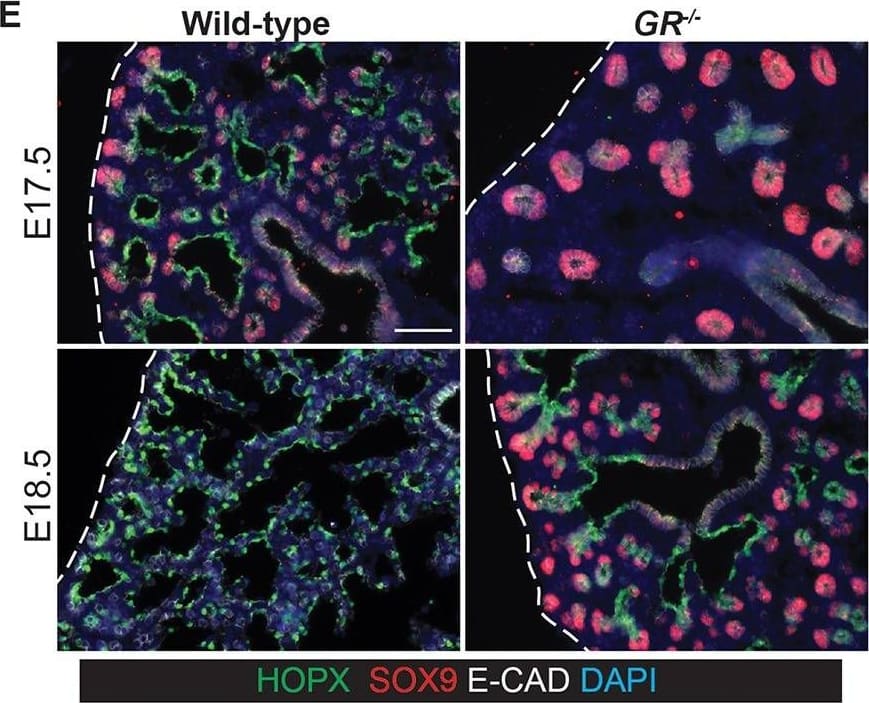
Glucocorticoid signalling is sufficient, but not essential, to specify alveolar fate. (A) Experimental design: Tomato+ E12.5 or 16.5 tip or stalk was grafted into E12.5 host lung and grown with 50 nM Dx throughout culture. (B) Examples of alveolar-fated tip grafts stained for: green, LPCAT1 (alveolar fate); red, Tomato (graft); white, PDPN (basal and AT1 cells). Arrowheads, PDPN+ AT1 cells. (C) Split bar graph showing results from B. Each type of graft was analysed in at least three independent experiments. (D) E12.5 wild-type lungs were grown with or without Dx for up to 6 days; two independent experimental replicates. Note precocious expression of alveolar markers in the presence of Dx. Lungs cultured without Dx do express LPCAT1 from experimental day 5. Green, LPCAT1 (late tip progenitors and type 2 cells); red, SOX9 (tip progenitors). (E,F) Sections of GR−/− and GR+/+ sibling lungs at E17.5 and E18.5 stained for: green, HOPX (AT1 cells); red, SOX9 (tip progenitors); white, E-CAD (epithelium) (E), and: green, LPCAT1 (late tip progenitors and AT2 cells); red, LAMP3 (AT2 cells); magenta, PDPN (late tip progenitors and AT1 cells) (F). A total of five GR−/− and 5 GR+/+ sibling lungs from three independent litters were observed at both E17.5 and E18.5. Blue, DAPI. Dashed line, edge of lung. Scale bars: 100 μm in B; 50 μm in D-F. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27578791), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Human SOX9 Antibody by Immunohistochemistry
Evolution of alveolar epithelial gene expression patterns in the developing mouse lung. Sections of E15.5, 16.5, 17.5 and 18.5 wild-type mouse lungs stained for markers of differentiation. (A) Green, SOX2 (differentiating bronchioles); red, SOX9 (tips); white, LPCAT1 (tip cells from E16.5, then AT2 cells). (B) Green, CEBPA (sub-set of tip cells from E16.5, then AT2 cells); red, pro-SFTPC (embryonic epithelium, stronger from E16.5, later specific to AT2 cells). (C) Green, pro-SFTPC (stronger from E16.5, later specific to AT2 cells); red, LAMP3 (rare tip cells; AT2 cells); magenta, PDPN (tip cells from E16.5, then AT1 cells). (D) Green, LPCAT1 (tip cells from E16.5, then AT2 cells); red, LAMP3 (rare tip cells; AT2 cells); magenta, PDPN (tip cells from E16.5, then AT1 cells). (E) Green, HOPX (stalk cells from E16.5, AT1 cells); red, SOX9 (tip cells); white, E-CAD (epithelial cells). (F) Green, SOX2 (differentiating bronchioles); red, SOX9 (tips); white, HOPX (stalk cells from E16.5, AT1 cells). (G) Green, HOPX (stalk cells from E16.5, AT1 cells); red, LPCAT1 (tip cells from E16.5, then AT2 cells). Arrows, LPCAT1+ HOPX+ cells; arrowheads, LPCAT1+ HOPX− cells. Blue, DAPI (nuclei). Dashed line, edge of lung. Scale bars: 50 μm in A-F, 20 μm in G and insets. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27578791), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Human SOX9 Antibody by Immunohistochemistry
Glucocorticoid signalling is sufficient, but not essential, to specify alveolar fate. (A) Experimental design: Tomato+ E12.5 or 16.5 tip or stalk was grafted into E12.5 host lung and grown with 50 nM Dx throughout culture. (B) Examples of alveolar-fated tip grafts stained for: green, LPCAT1 (alveolar fate); red, Tomato (graft); white, PDPN (basal and AT1 cells). Arrowheads, PDPN+ AT1 cells. (C) Split bar graph showing results from B. Each type of graft was analysed in at least three independent experiments. (D) E12.5 wild-type lungs were grown with or without Dx for up to 6 days; two independent experimental replicates. Note precocious expression of alveolar markers in the presence of Dx. Lungs cultured without Dx do express LPCAT1 from experimental day 5. Green, LPCAT1 (late tip progenitors and type 2 cells); red, SOX9 (tip progenitors). (E,F) Sections of GR−/− and GR+/+ sibling lungs at E17.5 and E18.5 stained for: green, HOPX (AT1 cells); red, SOX9 (tip progenitors); white, E-CAD (epithelium) (E), and: green, LPCAT1 (late tip progenitors and AT2 cells); red, LAMP3 (AT2 cells); magenta, PDPN (late tip progenitors and AT1 cells) (F). A total of five GR−/− and 5 GR+/+ sibling lungs from three independent litters were observed at both E17.5 and E18.5. Blue, DAPI. Dashed line, edge of lung. Scale bars: 100 μm in B; 50 μm in D-F. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27578791), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Human SOX9 Antibody by Immunohistochemistry
Evolution of alveolar epithelial gene expression patterns in the developing mouse lung. Sections of E15.5, 16.5, 17.5 and 18.5 wild-type mouse lungs stained for markers of differentiation. (A) Green, SOX2 (differentiating bronchioles); red, SOX9 (tips); white, LPCAT1 (tip cells from E16.5, then AT2 cells). (B) Green, CEBPA (sub-set of tip cells from E16.5, then AT2 cells); red, pro-SFTPC (embryonic epithelium, stronger from E16.5, later specific to AT2 cells). (C) Green, pro-SFTPC (stronger from E16.5, later specific to AT2 cells); red, LAMP3 (rare tip cells; AT2 cells); magenta, PDPN (tip cells from E16.5, then AT1 cells). (D) Green, LPCAT1 (tip cells from E16.5, then AT2 cells); red, LAMP3 (rare tip cells; AT2 cells); magenta, PDPN (tip cells from E16.5, then AT1 cells). (E) Green, HOPX (stalk cells from E16.5, AT1 cells); red, SOX9 (tip cells); white, E-CAD (epithelial cells). (F) Green, SOX2 (differentiating bronchioles); red, SOX9 (tips); white, HOPX (stalk cells from E16.5, AT1 cells). (G) Green, HOPX (stalk cells from E16.5, AT1 cells); red, LPCAT1 (tip cells from E16.5, then AT2 cells). Arrows, LPCAT1+ HOPX+ cells; arrowheads, LPCAT1+ HOPX− cells. Blue, DAPI (nuclei). Dashed line, edge of lung. Scale bars: 50 μm in A-F, 20 μm in G and insets. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27578791), licensed under a CC-BY license. Not internally tested by R&D Systems.
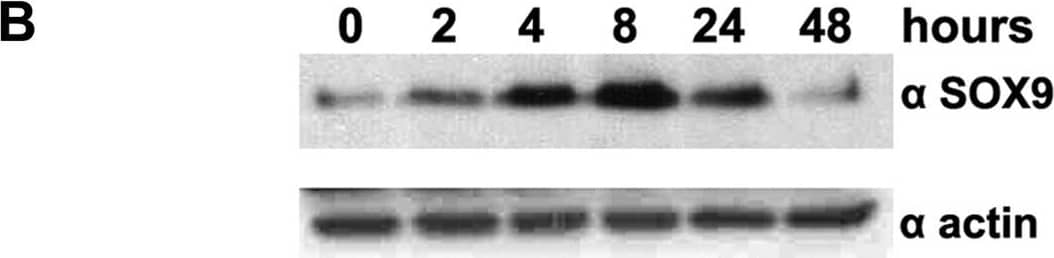
Detection of SOX9 by Western Blot
Validation of stem cell associated proteins after HGF stimulation.A, FACS demonstrated up-regulation of CD49b (240%), CD49f (22%), CD44 (26%), together with down-regulation of CD24 (−69%) after HGF stimulation. Thin gray line represents labelling with secondary antibody only (negative control), thin black line DU145 without HGF, and thick black line DU145 with HGF. B, Western blot showed enhancement of SOX9 expression upon HGF stimulation from 2 to 24 hours. C, FACS demonstrated induction of double-labelled CD44+/CD24− DU145 cells (control 8% versus HGF 26%). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/22110593), licensed under a CC-BY license. Not internally tested by R&D Systems.