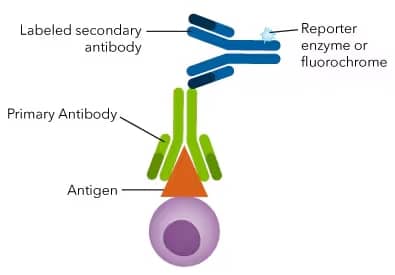
A conjugated antibody (also known as a tagged, loaded or labeled antibody) is one which has been modified by the chemical addition of an enzyme, toxin, or inorganic compound. Modern immunoassay techniques make extensive use of antibodies conjugated with biotin or fluorescent dyes.
For the benefits of those who are new to this area, we thought it would be a good idea to provide a synopsis on what these proteins actually are, how they work, and how they are used.
Conjugated antisera are used in many research applications, from Western blotting to in vivo cellular analysis. Researchers are looking for two things – a strong signal and specificity to particular antigens. At Bio-Techne we pride ourselves on having an extensive catalog of highly specific products, including antibodies tagged with the very latest and most effective fluorophores.
Today, thousands of antibodies have been conjugated for detection of a wide range of antigen targets in a wide variety of assay formats.
| ELISA | biotin, enzymes |
| ELISpot | biotin, enzymes, fluorochromes |
| Western Blot | biotin, enzymes, epitope tags, fluorochromes |
| Flow Cytometry | fluorochromes, biotin, enzymes |
| Mass Cytometry | heavy metals |
| Luminex® Assays | fluorochromes, biotin, enzymes |
| Immunohistochemistry | biotin, enzymes |
| Immunocytochemistry | biotin, enzymes |
| Immunofluorescence | fluorochromes, biotin |
| Cell Sorting | fluorochromes, biotin |
Although antibody suppliers like us at Bio-Techne manufacture our conjugates solely for non-clinical use, monoclonal antibody conjugates are also extensively used in cancer treatment. By locking onto specific antigens in cancer cells, they can specifically deliver drugs, radiotherapy, and toxins without damaging surrounding tissues. These antibody conjugates can also be used for in vivo tumor imaging.
Conjugation of Primary or Secondary Antibodies?
The label in an immunoassay provides either ‘direct’ or ‘indirect’ detection of the antigen. With direct detection, the label is attached to the primary antibody that binds the antigen itself. Alternatively, with indirect detection, the label is attached to a secondary antibody, which then binds to the primary antibody.
With direct detection, the covalent attachment of the label to the primary antibody means that only a single incubation step with the antigen is required. Both functions of antibody binding and signal generation are accomplished with a single reagent. Only a single round of wash steps is required, as opposed to two rounds of incubation and wash steps with indirect detection. This simplified assay protocol saves time, decreases assay variability, and improves data quality.
In the case of indirect detection, the assay comprises two distinct parts. First, the primary antibody is incubated with the sample. Next, the labeled secondary reagent is added which binds to the primary antibody and generates the actual signal for the assay. Using a conjugated secondary antibody can increase assay sensitivity by signal amplification and not risk damaging the antigen recognition by the primary antibody.
Fluorescent Conjugates
Conjugating antibodies with fluorescent compounds (fluorophores or fluorochromes) allows antibody detection with optical equipment. Fluorescent conjugated antibodies can be used in many different techniques including flow cytometry, fluorescence activated cell sorting (FACS), immunofluorescence, and Western blot. Individual fluorophores emit light at characteristic wavelengths (emission) after laser stimulation of the correct wavelength (excitation). By using different fluorescently conjugated antibodies, you can detect multiple targets at once (multiplexing) if the instrumentation can resolve the different emission signals.
Bio-Techne offers a wide variety of antibodies that are directly conjugated to a fluorescent molecule.
Mass Cytometry (CyTOF) Conjugates
Mass cytometry, also known as cytometry by time-of-flight (CyTOF®), combines the techniques of flow cytometry and elemental mass spectrometry. CyTOF antibodies are conjugated to rare earth metal isotopes instead of fluorochromes. The unique mass signatures of these metals enables time-of-flight mass spectrometers (TOFMS) to accurately discriminate over 40 targets per cell in comparison to just over 20 parameters using conventional flow cytometry. CyTOF analysis is significantly less sensitive than fluorescence analysis, but it does not require the use of isotype controls.
Biotin and Epitope Tags
These molecules serve as affinity tags for binding of another assay reagent for detection. They are conjugated to primary antibodies and followed by a secondary reagent that itself is conjugated to provide the assay readout. Biotinylated antibody conjugates are detected by streptavidin (SA) that has an extremely high affinity for biotin and also serves to amplify the signal for improved detection of rare target epitopes. Epitope-tagged antibodies are detected by a secondary antibody that recognizes the epitope tag itself. In either of these cases, the secondary reagent (streptavidin or antibody) is conjugated to a fluorophore or to an enzyme for colorimetric detection.
Enzyme Conjugates
In addition to fluorescent conjugates, antibodies can also be conjugated to enzymes such as horseradish peroxidase (HRP) and alkaline phosphatase (AP). These enzymes convert chromogenic substrates into either a soluble colored compound or an insoluble colored precipitate that remains localized to the site of the antibody-antigen complex. The most commonly used enzymes for antibody conjugation are alkaline phosphatase (AP) which acts on NPP or NADPH, and horseradish peroxidase (HRP) which acts on DAB, TMB, or chemiluminescent (ECL) substrates. Insoluble color formation and ECL are valuable for IHC and Western blot applications, while soluble color formation is ideal for ELISA.
Additional Resources
Primary and Secondary Antibodies
Find Conjugated Primary Antibodies
Recombinant Monoclonal Antibodies
Fluorescent Conjugates
Multicolor Flow Cytometry Panels
Interactive Cell Marker Tool at R&D Systems
Tandem Dye Conjugated Antibodies FAQs
CyTOF Conjugates
Read More About Mass Cytometry
Biotin and Epitope Tags
Enzyme Conjugates
Find HRP-Conjugated Antibodies
Technology Brief on HRP-Polymer Conjugate
VisUCyte™ HRP Polymer Conjugates and Staining Kits
A Guide to Western Blot Detection: Chemiluminescence Versus Fluorescence
Conjugation Kits and Methods
Explore Antibody Conjugation Illustrated Assay
Lightning-Link™ Antibody Labeling Kits
Conjugation Protocol for Amine Reactive CoraFluor™ Reagents at Tocris
Conjugation Protocol for Amine Reactive Dyes at Tocris
Conjugation Protocol for Thiol Reactive (Maleimide) Dyes at Tocris
Detection and Visualization of Antibody Binding at R&D Systems
Antibody Conjugation Troubleshooting
CyTOF is a registered trademark of Standard BioTools.
Luminex is a registered trademark of Luminex Corporattion.