The human form of microtubule-associated protein light chain 3 (LC3) is expressed as 3 splice variants LC3A, LC3B, and LC3C.1 LC3B is a subunit of the MAP1A and MAP1B microtubule-binding proteins and plays a central role in autophagosome membrane structure. This ubiquitin-like modifier is known to be involved in early stages of autophagosome formation and specifically with phagophore membrane elongation. LC3B also interacts with autophagy receptors such as p62/SQSTM1 and NBR1 during substrate selection for autophagic degradation.2
Researchers using LC3B knock out mice to determine if LC3 is required for autophagy found that the mice do develop normally, likely due to a compensatory autophagy mechanism by other members of the MAP1LC3 subfamily.3
During early stages of autophagosome formation, the cytosolic form of LC3, known as LC3-I, becomes conjugated to the head of a lipid via a series of ubiquitin-like reactions. This lipid modified form of LC3, known as LC3-II, is then believed to be recruited for autophagosome membrane expansion and fusion. Detecting the conversion of LC3-I (~19 kDa) to LC3-II (~17 kDa) is considered a useful, if not definitive4, autophagy marker in mammalian cells. Unlike LC3A and LC3C however, LC3B does not undergo C-terminal cleavage and exists in a single modified form with an essential site for post-translation modification at Lys-122 rather than the conserved Gly-120 of the other two variants.1
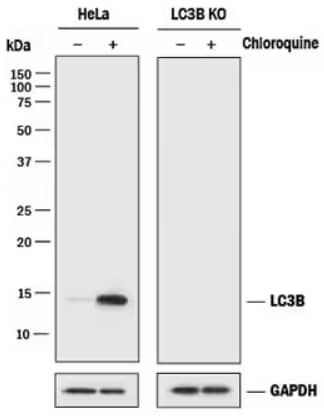
Genetic Strategies Validation. Western Blot analysis showing lysates from HeLa human cervical epithelial carcinoma parental cell line and LC3B knockout (KO) HeLa cell line that were either untreated (-) or treated (+) with Chloroquine. The membrane was probed with Rabbit Anti-LC3B Monoclonal Antibody (1251A) (Catalog # NBP2-46892) and detected using HRP-conjugated Anti-Rabbit IgG Secondary Antibody (Catalog # HAF008). A specific band for LC3B is shown at ~ 15 kDa in the parental HeLa cell line, and is increased with Chloroquine treatment, but is absent in the KO cell line. GAPDH was used as a loading control.
Antibodies to all three LC3 splice variants are widely used as markers of autophagosomes. For example, our LC3B/MAP1LC3B Antibody [NB100-2220] has been cited for use as an autophagosome marker in over 1300 published research articles in a wide variety of applications. In one study, researchers used Bio-Techne's LC3B Antibodies [NB100-2220] and [NB600-1384] and recombinant proteins LC3A and LC3B to determine the distinct sub-cellular expression patterns of LC3B.5 In another study, the LC3B antibody [NB100-2220] was used to monitor autophagy during exercise and insulin stimulation in skeletal muscle.6
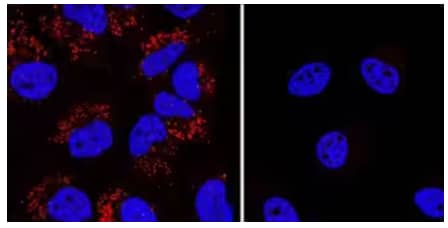
Genetic Strategies Validation. Immunocytochemical/Immunofluorescence analysis of LC3B expression in chloroquine treated HeLa cells (left) but not in LC3B-knockout (KO) HeLa cells (right) using Rabbit Anti-LC3B Polyclonal Antibody (Catalog # NB100-2220) followed by staining with NorthernLights™ 557-conjugated Anti-Ratbit IgG Secondary Antibody (Catalog # NL004) (red) and nuclei counterstaining with DAPI (blue).
In many studies, researchers have used Bio-Techne's fluorescent conjugated LC3B/MAP1LC3B antibodies for autophagosome labeling in immunofluorescence and flow cytometry applications. For instance, researchers used the DyLight-conjugated LC3B antibody [NB100-2220G] to report a link between AKT mediated glycolytic metabolism and autophagy.7 Additionally, our AlexaFlour-conjugated LC3B antibody [NB100-2220AF488] on cultured monocytes to label autophagosomes.8
|
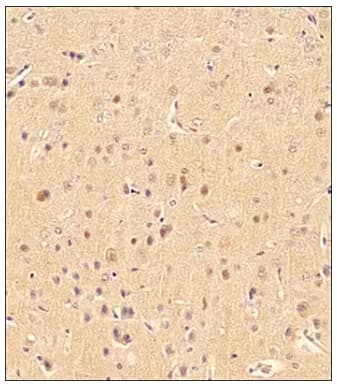
Immunohistochemical analysis of LC3B in formalin-fixed, paraffin-embedded mouse brain tissue sections using Rabbit Anti-LC3B Polyclonal Antibody (Catalog # NB100-2220) and detected using HRP-conjugated Secondary Antibody with DAB reagent and nuclei counterstaining with hematoxylin. The tissue shows low to moderate levels of LC3B antibody staining in the cytoplasm of glial cells and stronger staining in the cytoplasm of neurons. |
|
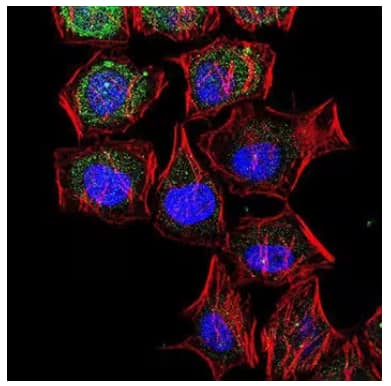
Immunocytochemical confocal image analysis of LC3B expression in HeLa cells using Rabbit Anti-LC3B Polyclonal Antibody (Catalog # NB600-1384) and detected using Alexa Fluor 488-conjugated IgG Secondary Antibody (green). Actin filaments (red) were detected with Alexa Fluor 568 phalloidin and nuclear staining with DAPI (blue). |
-
He, H et al. (2003) Post-translational modifications of three members of the human MAP1LC3 family and detection of a novel type of modification for MAP1LC3B J Biol Chem 278:29278-29287. PMID: 12740394.
-
Johansen, T. & Lamark, T. (2011) Selective autophagy mediated by autophagic adapter proteins Autophagy 7:279-96. PMID: 21189453.
-
Cann GM et al. (2008) Developmental expression of LC3α and β: Absence of fibronectin or autophagy phenotype in LC3β knockout mice Dev Dyn 237:187-95. PMID: 18069693.
-
Giménez-Xavier, P. et al. (2008) LC3-I conversion to LC3-II does not necessarily result in complete autophagy Int J Mol Med 2008:781-5. PMID: 19020776.
-
Koukourakis MI et al. (2015) Autophagosome Proteins LC3A, LC3B and LC3C Have Distinct Subcellular Distribution Kinetics and Expression in Cancer Cell Lines PLoS ONE PMID: 263378792.
-
Fritzen, AM et al. (2016) Regulation of autophagy in human skeletal muscle - Effects of exercise, exercise training and insulin stimulation J Physiol 594:745-61. PMID: 26614120.
-
Matta, SK. & Kumar D. (2015) AKT mediated glycolytic shift regulates autophagy in classically activated macrophages Int Int. J. Biochem. Cell Biol PMID: 26222186.
-
Yuan, R. et al. (2015) Low-grade inflammatory polarization of monocytes impairs wound healing J. Pathol PMID: 26690561.