Histone H2AX [p Ser139] Antibody (RM224)
Novus Biologicals, part of Bio-Techne | Catalog # NBP3-18533
Recombinant Monoclonal

Conjugate
Catalog #
Forumulation
Catalog #
Key Product Details
Validated by
Biological Validation
Species Reactivity
Human, Vertebrate
Applications
ELISA, Immunocytochemistry/ Immunofluorescence, Multiplex Immunoassay, Western Blot
Label
Unconjugated
Antibody Source
Recombinant Monoclonal Rabbit IgG Clone # RM224
Concentration
1 mg/ml
Product Specifications
Immunogen
A phospho-peptide corresponding to Histone H2AX [Ser139]
Modification
p Ser139
Specificity
This antibody reacts to Histone H2AX only when phosphorylated at serine 139. No cross reactivity with other phosphorylated histones.
Clonality
Monoclonal
Host
Rabbit
Isotype
IgG
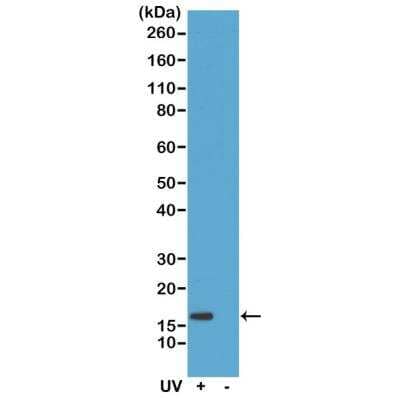
Scientific Data Images for Histone H2AX [p Ser139] Antibody (RM224)
Immunocytochemistry/Immunofluorescence: Histone H2AX [p Ser 119] Antibody (RM224) [NBP3-18533] - Immunocytochemistry of HeLa cells using NBP3-18533 (red). Actin filaments have been labeled with fluorescein phalloidin (green).
Applications for Histone H2AX [p Ser139] Antibody (RM224)
Application
Recommended Usage
ELISA
0.2 ug/mL - 1 ug/mL
Immunocytochemistry/ Immunofluorescence
0.5 ug/mL - 2 ug/mL
Multiplex Immunoassay
0.1 ug/mL - 1 ug/mL
Western Blot
0.5 ug/mL - 2 ug/mL
Formulation, Preparation, and Storage
Purification
Protein A purified
Formulation
50% Glycerol/PBS, 1% BSA
Preservative
0.09% Sodium Azide
Concentration
1 mg/ml
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below.
Stability & Storage
Store at -20C.
Background: Histone H2AX
Alternate Names
H2AFX
Gene Symbol
H2AX
Additional Histone H2AX Products
Product Documents for Histone H2AX [p Ser139] Antibody (RM224)
Product Specific Notices for Histone H2AX [p Ser139] Antibody (RM224)
This product is for research use only and is not approved for use in humans or in clinical diagnosis. Primary Antibodies are guaranteed for 1 year from date of receipt.
Loading...
Loading...
Loading...
Loading...
