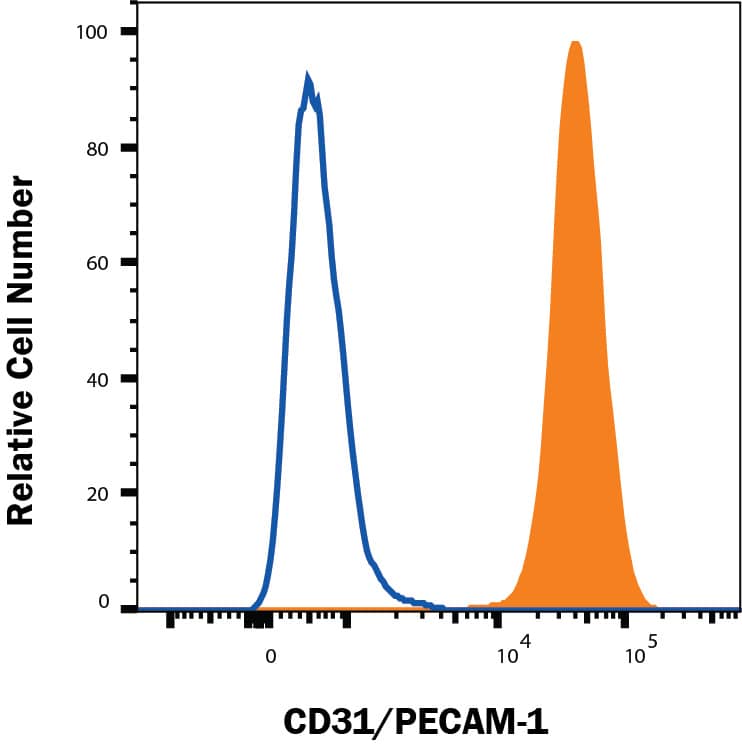
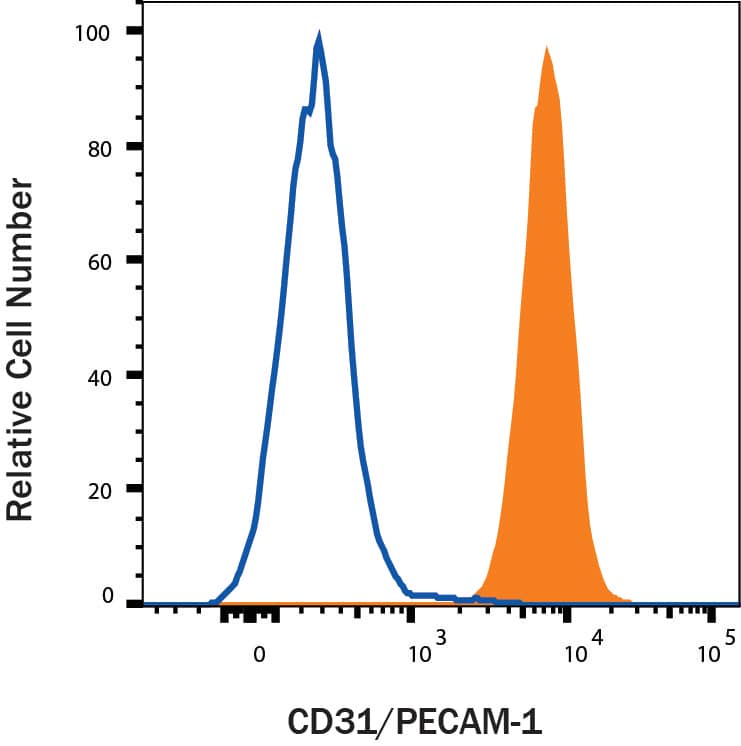
Detection of CD31/PECAM‑1 in Rat Splenocytes by Flow Cytometry.
Rat splenocytes were stained with Goat Anti-Human/Mouse/Rat CD31/PECAM-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628, filled histogram) or isotype control antibody (
AB-108-C, open histogram), followed by Allophycocyanin-conjugated Anti-Goat IgG Secondary Antibody (
F0108).
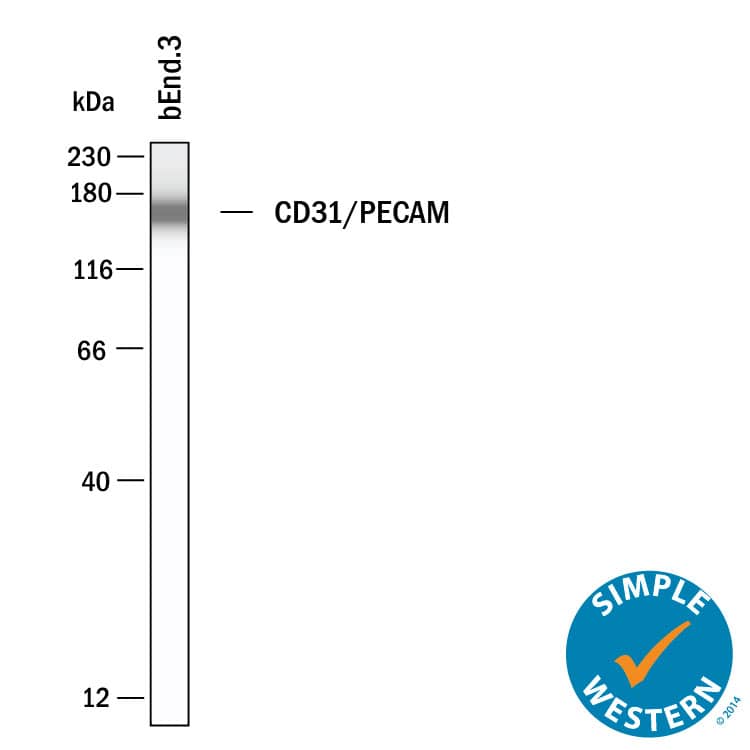
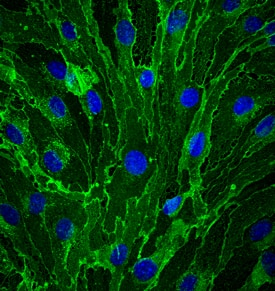
CD31/PECAM‑1 in bEnd.3 Mouse Cell Line.
CD31/PECAM-1 was detected in immersion fixed bEnd.3 mouse endothelioma cell line using Goat Anti-Human/Mouse/Rat CD31/PECAM-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 493-conjugated Anti-Goat IgG Secondary Antibody (green;
NL003) and counterstained with DAPI (blue). Specific staining was localized to cell membrane. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
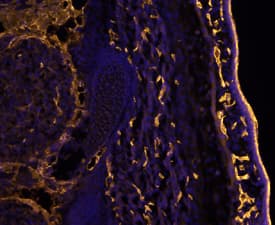
CD31/PECAM‑1 in Mouse Embryo.
CD31/PECAM-1 was detected in immersion fixed frozen sections of mouse embryo (E13.5) using Goat Anti-Human/Mouse/Rat CD31/PECAM-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628) at 10 µg/mL overnight at 4 °C. Tissue was stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (yellow;
NL001) and counterstained with DAPI (blue). Specific staining was localized to developing endothelium. View our protocol for Fluorescent IHC Staining of Frozen Tissue Sections.
CD31/PECAM‑1 in Mouse Embryo.
CD31/PECAM-1 was detected in immersion fixed frozen sections of mouse embryo (14 d.p.c.) using Goat Anti-Human/Mouse/Rat CD31/PECAM-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628) at 10 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (
VC004). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to developing guts. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
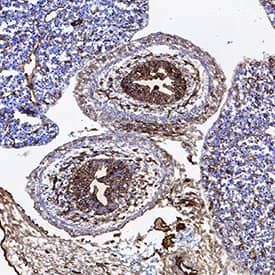
CD31/PECAM-1 in Rat Heart.
CD31/PECAM-1 was detected in immersion fixed paraffin-embedded sections of rat heart using Goat Anti-Human/Mouse/Rat CD31/PECAM-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628) at 3 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Mouse IgG VisUCyte™ HRP Polymer Antibody (
VC001). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (
CTS013). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to endothelial cells in vasculature.
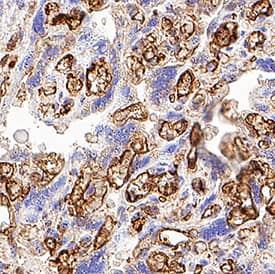
Detection of CD31/PECAM-1 in Human Placenta.
CD31/PECAM-1 was detected in immersion fixed paraffin-embedded sections of Human Placenta using Goat Anti-Human/Mouse/Rat CD31/PECAM-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628) at 15 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (Catalog #
VC004). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using VisUCyte Antigen Retrieval Reagent-Basic (Catalog #
VCTS021). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to endothelial cells in chorionic villi. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
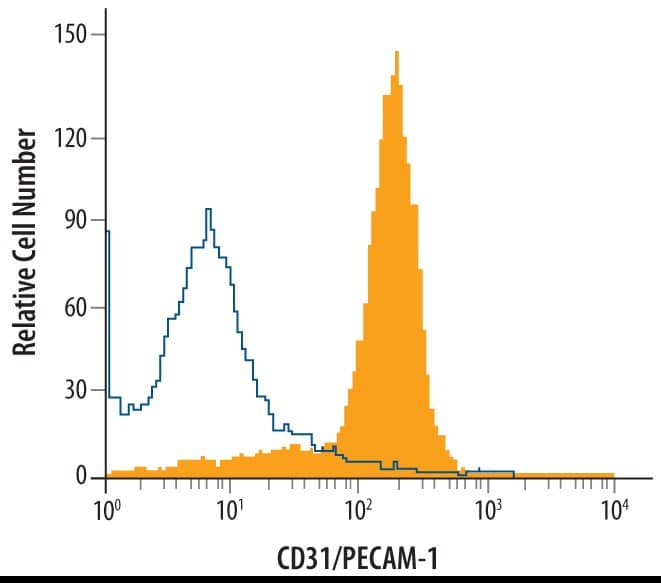
Detection of CD31/PECAM-1 in HUVEC cells by Flow Cytometry.
HUVEC cells were stained with Goat Anti-Human/Mouse/Rat CD31/PECAM-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628, filled histogram) or isotype control antibody (Catalog #
AB-108-C, open histogram), followed by Phycoerythrin-conjugated Anti-Goat IgG Secondary Antibody (Catalog #
F0107). View our protocol for Staining Membrane-associated Proteins.
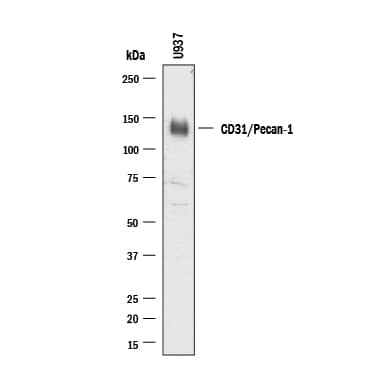
Detection of Human CD31/PECAM-1 by Western Blot.
Western blot shows lysates of U937 human histiocytic lymphoma cell line. PVDF membrane was probed with 0.5 µg/ml of Goat Anti-Human/Mouse/Rat CD31/PECAM-1 Antigen Affinity-purified Polyclonal Antibody (
AF3628) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog #
HAF017). A specific band was detected for CD31/PECAM-1 at approximately 130 kDa (as indicated). This experiment was conducted under reducing conditions and using Western Blot Buffer Group 1.
Detection of Mouse CD31/PECAM-1 by Immunohistochemistry
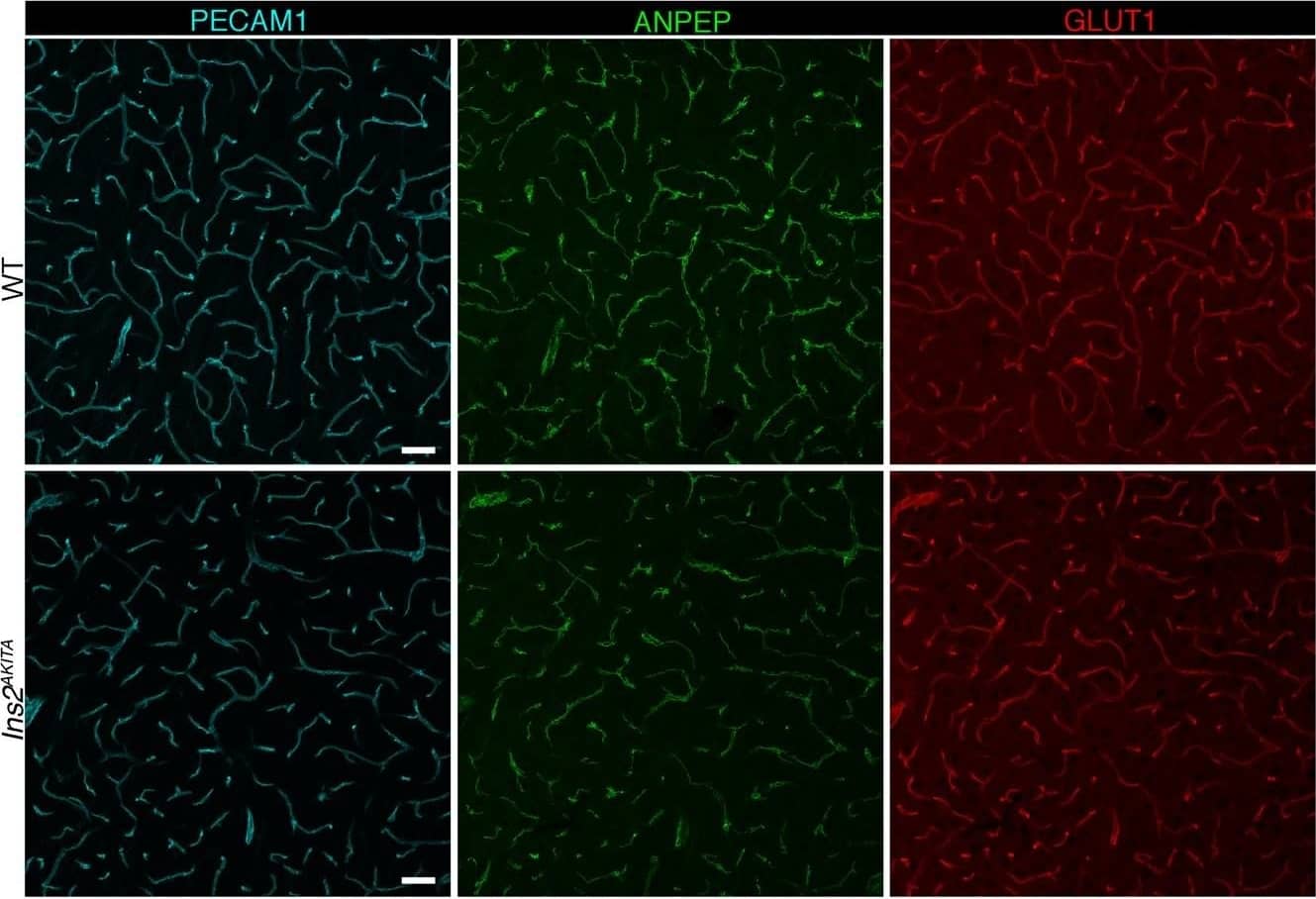
Representative image of immunostaining corresponding to the glucose transporter protein SLC2A1 (GLUT1) in 30 week-old Ins2AKITA and WT cerebral cortex. Mural cells (ANPEP, green), glucose transporter 1 (GLUT1, red), endothelium (PECAM1, cyan). n = 2, scale bar 50 µm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30498224), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human CD31/PECAM-1 by Western Blot
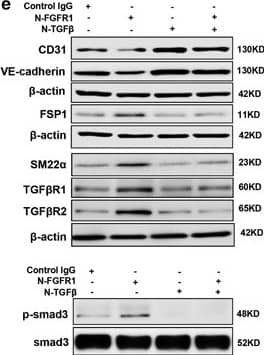
AcSDKP suppresses TGF beta/smad signaling and EndMT through the FGFR1/FRS2 pathway. (a) HMVECs were treated with N-FGFR1 for 48 h, and the FGFR1, TGF betaR1 and TGF betaR2 protein levels were analyzed by western blot. (b) HMVECs were treated with TGF beta2 in the presence or absence of N-FGFR1 for 15 min with or without AcSDKP preincubation. The p-smad3 and TGF betaR1 protein levels were analyzed by western blot. Densitometric analysis of the p-smad3/smad3 and TGF betaR1/ beta-actin levels (n=3) in each group was performed. (c) HMVECs were incubated with either N-FGFR1 in the presence or absence of TGF beta2 for 48 h with or without preincubation with AcSDKP for 2 h or with N-FGFR1 in the presence or absence of TGF beta2 for 48 h with or without 24 h of incubation with FGF2 (50 ng/ml). The CD31, SM22 alpha, FSP1 and alpha-SMA protein levels were analyzed by western blot. (d) HMVECs were transfected with FRS2 siRNA (100 nM) for 48 h with or without AcSDKP preincubation. The VE-cadherin, FSP1, vimentin, SM22 alpha and p-smad3 levels were analyzed by western blot. (e) HMVECs were treated with N-FGFR1 for 48 h or 15 min in the presence or absence of N-TGF beta (1, 2, 3) (1.0 μg/ml). The CD31, VE-cadherin, SM22 alpha, FSP1, TGF betaR1, TGF betaR2 and p-smad3 levels were analyzed by western blot Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28771231), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse CD31/PECAM-1 by Immunocytochemistry/Immunofluorescence
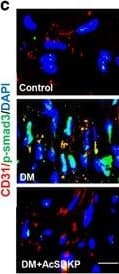
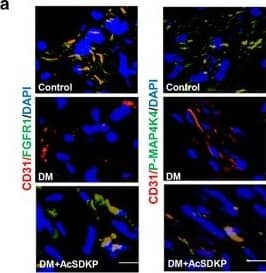
AcSDKP inhibits TGF beta/smad signaling and EndMT and restores the FGFR1 and P-MAP4K4 levels in diabetic hearts. (a) Immunofluorescence microscopy analysis of CD31/FGFR1 and CD31/P-MAP4K4 in the heart tissues from each group of mice. The scale bar is 60 μm in each panel. The CD31 and FGFR1 double-labeled cells and the CD31 and P-MAP4K4 double-labeled cells in each visual field were assessed by fluorescence microscopy and quantified. For each section, images from six different fields of view at × 400 magnification were evaluated. (b and c) Immunofluorescence microscopy analysis of CD31/ alpha-SMA, VE-cadherin /SM22 alpha and CD31/p-smad3 expression levels in the heart tissues from each group of mice. The scale bar is 60 μm in each panel. The CD31 and alpha-SMA double-labeled cells, the VE-cadherin and SM22 alpha double-labeled cells and the CD31 and p-smad3 double-labeled cells in each visual field were analyzed by fluorescence microscopy and quantified. For each section, images from six different fields of view at × 400 magnification were evaluated. Four mice from each group were analyzed. (d) Western blot analysis of the FGFR1, P-MAP4K4, TGF beta1, TGF beta2 and TGF beta3 levels in cardiac tissues. A representative blot from four independent experiments was shown. The densitometric analysis of western blot data was presented (n=4). The diabetic mice are abbreviated as DM in the figure Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28771231), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human CD31/PECAM-1 by Western Blot
AcSDKP suppresses TGF beta/smad signaling and EndMT through the FGFR1/FRS2 pathway. (a) HMVECs were treated with N-FGFR1 for 48 h, and the FGFR1, TGF betaR1 and TGF betaR2 protein levels were analyzed by western blot. (b) HMVECs were treated with TGF beta2 in the presence or absence of N-FGFR1 for 15 min with or without AcSDKP preincubation. The p-smad3 and TGF betaR1 protein levels were analyzed by western blot. Densitometric analysis of the p-smad3/smad3 and TGF betaR1/ beta-actin levels (n=3) in each group was performed. (c) HMVECs were incubated with either N-FGFR1 in the presence or absence of TGF beta2 for 48 h with or without preincubation with AcSDKP for 2 h or with N-FGFR1 in the presence or absence of TGF beta2 for 48 h with or without 24 h of incubation with FGF2 (50 ng/ml). The CD31, SM22 alpha, FSP1 and alpha-SMA protein levels were analyzed by western blot. (d) HMVECs were transfected with FRS2 siRNA (100 nM) for 48 h with or without AcSDKP preincubation. The VE-cadherin, FSP1, vimentin, SM22 alpha and p-smad3 levels were analyzed by western blot. (e) HMVECs were treated with N-FGFR1 for 48 h or 15 min in the presence or absence of N-TGF beta (1, 2, 3) (1.0 μg/ml). The CD31, VE-cadherin, SM22 alpha, FSP1, TGF betaR1, TGF betaR2 and p-smad3 levels were analyzed by western blot Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28771231), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse CD31/PECAM-1 by Immunocytochemistry/Immunofluorescence
AcSDKP inhibits TGF beta/smad signaling and EndMT and restores the FGFR1 and P-MAP4K4 levels in diabetic hearts. (a) Immunofluorescence microscopy analysis of CD31/FGFR1 and CD31/P-MAP4K4 in the heart tissues from each group of mice. The scale bar is 60 μm in each panel. The CD31 and FGFR1 double-labeled cells and the CD31 and P-MAP4K4 double-labeled cells in each visual field were assessed by fluorescence microscopy and quantified. For each section, images from six different fields of view at × 400 magnification were evaluated. (b and c) Immunofluorescence microscopy analysis of CD31/ alpha-SMA, VE-cadherin /SM22 alpha and CD31/p-smad3 expression levels in the heart tissues from each group of mice. The scale bar is 60 μm in each panel. The CD31 and alpha-SMA double-labeled cells, the VE-cadherin and SM22 alpha double-labeled cells and the CD31 and p-smad3 double-labeled cells in each visual field were analyzed by fluorescence microscopy and quantified. For each section, images from six different fields of view at × 400 magnification were evaluated. Four mice from each group were analyzed. (d) Western blot analysis of the FGFR1, P-MAP4K4, TGF beta1, TGF beta2 and TGF beta3 levels in cardiac tissues. A representative blot from four independent experiments was shown. The densitometric analysis of western blot data was presented (n=4). The diabetic mice are abbreviated as DM in the figure Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28771231), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse CD31/PECAM-1 by Immunocytochemistry/Immunofluorescence
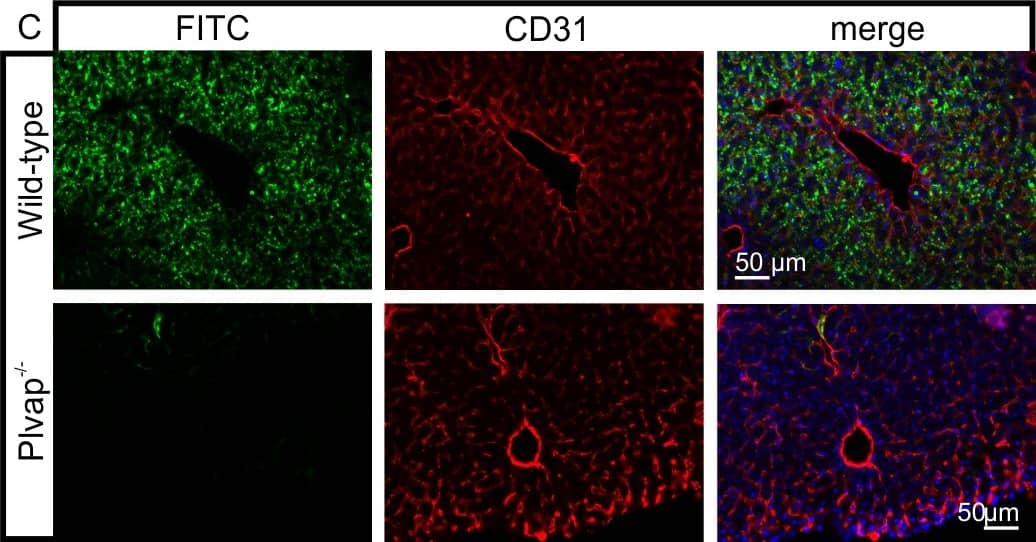
Diminished permeability of liver sinusoids in Plvap-deficient mice.Neither by immunohistochemistry with antibodies against CD31 (A) nor by light microscopy of 1 µm semi-thin sections (B, Richardson's stain) obvious differences are detected with regards to the overall orientation and the density of liver sinusoids between 3-week-old Plvap-/- mice and wild-type littermates. Sinusoids of Plvap-deficient mice show a higher number of macrophages in their lumen (white arrows) and focal areas with accumulations of mononuclear cells in Disse's space (black arrows). Lower panels in B show higher magnifications. C, After perfusion of a wild-type animal with FITC-dextran, a strong FITC-signal (green) throughout the liver is detected. Immunolabeling with CD31 (red) suggests that FITC-dextran molecules have accumulated in the space of Disse. In contrast, in the Plvap-deficient littermate, the signal for FITC-dextran is much weaker and barely detectable. Nuclear DNA is labeled with DAPI (blue). Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0115005), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse CD31/PECAM-1 by Immunohistochemistry
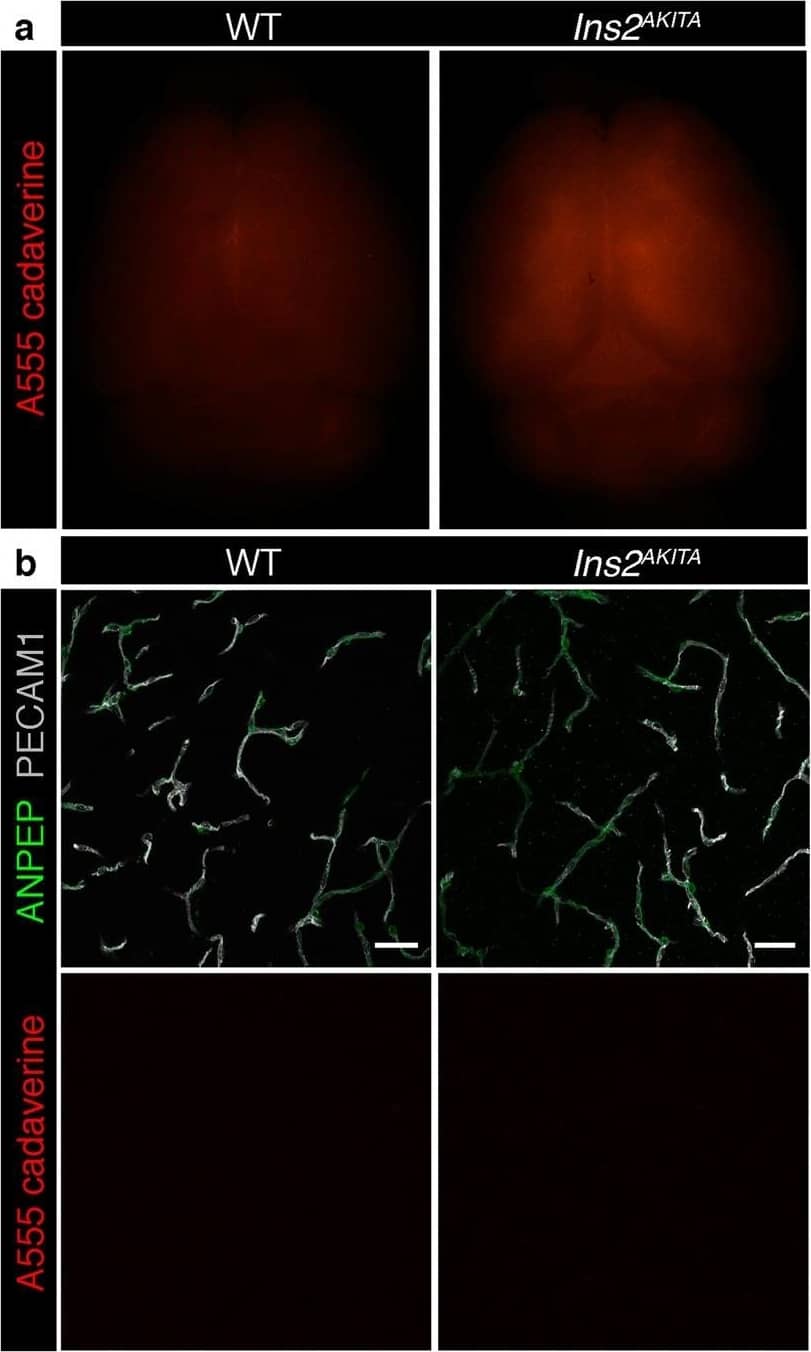
Blood-brain barrier permeability measurements in male Ins2AKITA and WT littermate controls. (a) Representative stereomicroscope fluorescence images of brains showing 1 kDa Alexa Fluor 555 cadaverine permeability in Ins2AKITA and WT after 2 h of dye circulation (n = 2). (b) Representative confocal images of coronal sections of 1 kDa Alexa Fluor 555 cadaverine injected mouse brains. ANPEP positive mural cells, green; PECAM1 positive vasculature, white. No Alexa Fluor 555 cadaverine leakage into brain parenchyma was observed either in Ins2AKITA or WT mice (n = 2, scale bar 30 μm). (c) Quantification of 1 kDa Alexa Fluor 555 cadaverine permeability in 26.5–32 week-old Ins2AKITA and WT mice after 2 h circulation (n = > 8, 3 independent experiments). y-axis shows the fold change in relative fluorescence units (RFU) per gram of brain tissue in relation to WT. (d) Quantification of 1 kDa Alexa Fluor 488 cadaverine permeability in 38 week-old Ins2AKITA and WT mice after 2 h circulation (n = 3). y-axis shows the fold change in relative fluorescence units (RFU) per gram of brain tissue in relation to WT. (e) Evans Blue dye permeability in 30 week-old Ins2AKITA and WT littermate control mice after overnight circulation (n = > 2). y-axis shows optical density (OD) at 620 nm per gram of tissue. PdgfbRet/Ret served as positive control for tracer leakage into the brain parenchyma. n.s., not significant, student’s t test. Data is presented as mean ± SEM. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30498224), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse CD31/PECAM-1 by Immunocytochemistry/Immunofluorescence
Diminished permeability of liver sinusoids in Plvap-deficient mice.Neither by immunohistochemistry with antibodies against CD31 (A) nor by light microscopy of 1 µm semi-thin sections (B, Richardson's stain) obvious differences are detected with regards to the overall orientation and the density of liver sinusoids between 3-week-old Plvap-/- mice and wild-type littermates. Sinusoids of Plvap-deficient mice show a higher number of macrophages in their lumen (white arrows) and focal areas with accumulations of mononuclear cells in Disse's space (black arrows). Lower panels in B show higher magnifications. C, After perfusion of a wild-type animal with FITC-dextran, a strong FITC-signal (green) throughout the liver is detected. Immunolabeling with CD31 (red) suggests that FITC-dextran molecules have accumulated in the space of Disse. In contrast, in the Plvap-deficient littermate, the signal for FITC-dextran is much weaker and barely detectable. Nuclear DNA is labeled with DAPI (blue). Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0115005), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse CD31/PECAM-1 by Immunocytochemistry/Immunofluorescence
AcSDKP inhibits TGF beta/smad signaling and EndMT and restores the FGFR1 and P-MAP4K4 levels in diabetic hearts. (a) Immunofluorescence microscopy analysis of CD31/FGFR1 and CD31/P-MAP4K4 in the heart tissues from each group of mice. The scale bar is 60 μm in each panel. The CD31 and FGFR1 double-labeled cells and the CD31 and P-MAP4K4 double-labeled cells in each visual field were assessed by fluorescence microscopy and quantified. For each section, images from six different fields of view at × 400 magnification were evaluated. (b and c) Immunofluorescence microscopy analysis of CD31/ alpha-SMA, VE-cadherin /SM22 alpha and CD31/p-smad3 expression levels in the heart tissues from each group of mice. The scale bar is 60 μm in each panel. The CD31 and alpha-SMA double-labeled cells, the VE-cadherin and SM22 alpha double-labeled cells and the CD31 and p-smad3 double-labeled cells in each visual field were analyzed by fluorescence microscopy and quantified. For each section, images from six different fields of view at × 400 magnification were evaluated. Four mice from each group were analyzed. (d) Western blot analysis of the FGFR1, P-MAP4K4, TGF beta1, TGF beta2 and TGF beta3 levels in cardiac tissues. A representative blot from four independent experiments was shown. The densitometric analysis of western blot data was presented (n=4). The diabetic mice are abbreviated as DM in the figure Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28771231), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse CD31/PECAM-1 by Immunocytochemistry/Immunofluorescence
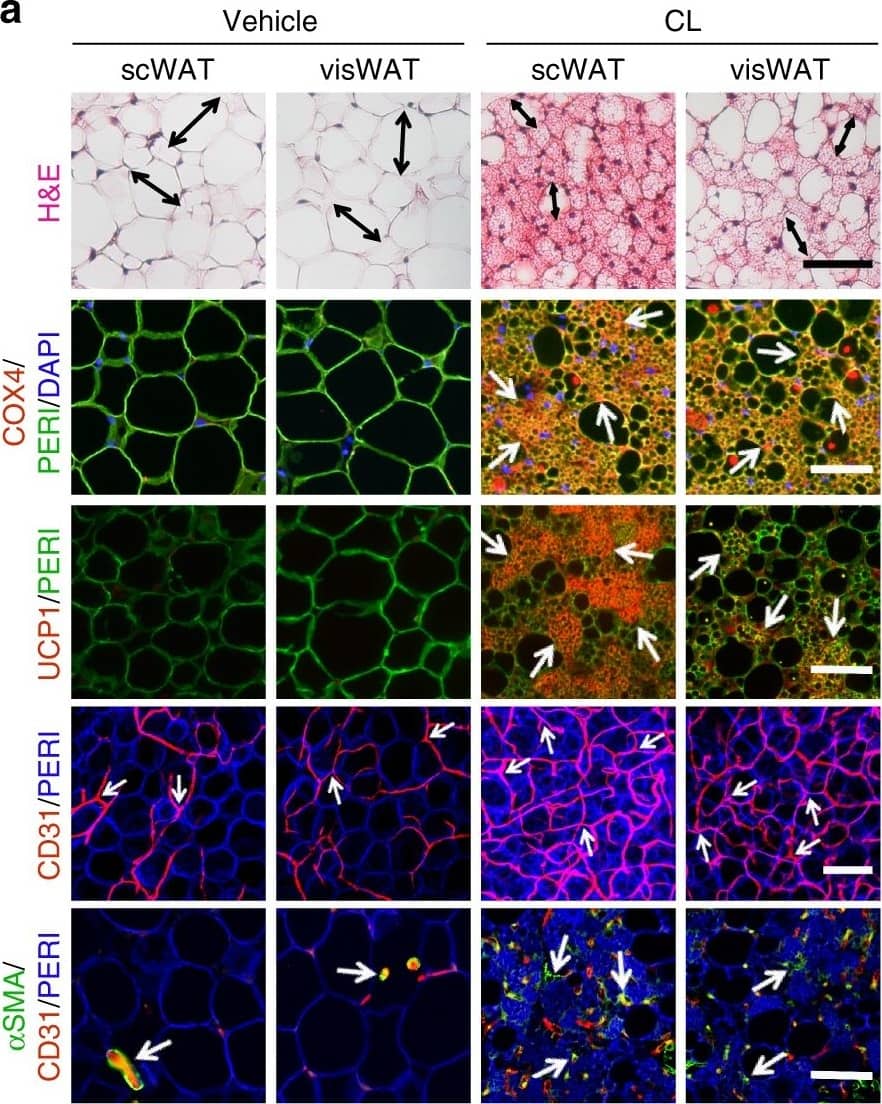
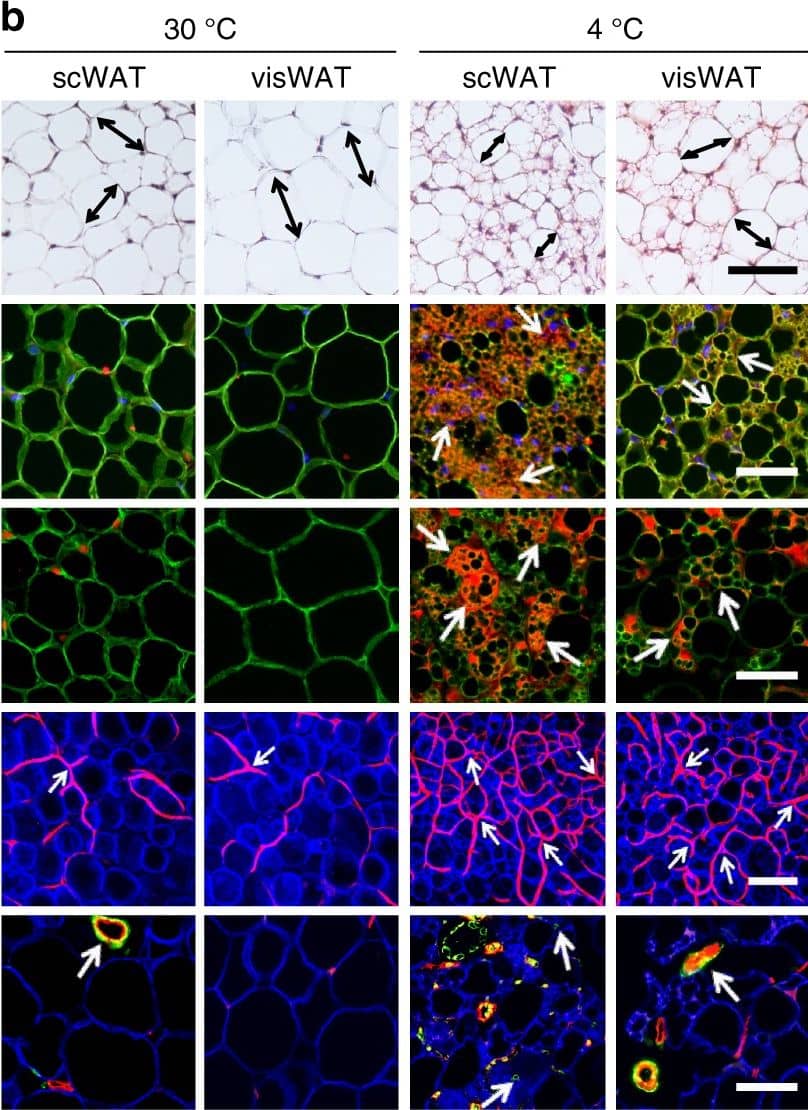
CL and low temperature lead to WAT browning, angiogenesis, and an increased number of myofibroblast-like cells. a, b Histological analysis of adipocyte morphology (H&E), adipocytes (PERI), mitochondria (COX4), uncoupling protein 1 (UCP1), blood vessels (CD31), and myofibroblast-like cells ( alphaSMA) in a 5-day CL-316243-treated scWAT and visWAT compared to vehicle-treated control. b Two-week 4 °C-treated scWAT and visWAT compared to 30 °C control. Double-headed arrows mark adipocyte diameter. Arrows point to respective positive signals. c–l Quantifications of adipocyte size and positive signals per field of COX4, UCP1, CD31, and alphaSMA of CL-316243- and vehicle-, and 30 °C- and 4 °C- treated scWATs and visWATs (>30 adipocytes per field; n = 10 random fields; n = 5 mice per group). PERI, perilipin; COX4, mitochondrial complex 4; UCP1, uncoupling protein 1. Scale bars, 100 μm. NS, not significant. *P < 0.05, **P < 0.01, and ***P < 0.001 by Student’s t-test. Data presented as mean ± s.e.m. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/s41467-017-02158-z), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse CD31/PECAM-1 by Immunocytochemistry/Immunofluorescence
CL and low temperature lead to WAT browning, angiogenesis, and an increased number of myofibroblast-like cells. a, b Histological analysis of adipocyte morphology (H&E), adipocytes (PERI), mitochondria (COX4), uncoupling protein 1 (UCP1), blood vessels (CD31), and myofibroblast-like cells ( alphaSMA) in a 5-day CL-316243-treated scWAT and visWAT compared to vehicle-treated control. b Two-week 4 °C-treated scWAT and visWAT compared to 30 °C control. Double-headed arrows mark adipocyte diameter. Arrows point to respective positive signals. c–l Quantifications of adipocyte size and positive signals per field of COX4, UCP1, CD31, and alphaSMA of CL-316243- and vehicle-, and 30 °C- and 4 °C- treated scWATs and visWATs (>30 adipocytes per field; n = 10 random fields; n = 5 mice per group). PERI, perilipin; COX4, mitochondrial complex 4; UCP1, uncoupling protein 1. Scale bars, 100 μm. NS, not significant. *P < 0.05, **P < 0.01, and ***P < 0.001 by Student’s t-test. Data presented as mean ± s.e.m. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/s41467-017-02158-z), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD31/PECAM-1 in Whole Blood Granulocytes by Flow Cytometry
Whole blood granulocytes were stained with Goat Anti-Human/Mouse/Rat CD31/PECAM-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628, filled histogram) or isotype control antibody (Catalog #
AB-108-C, open histogram) followed by Phycoerythrin-conjugated Anti-Goat IgG Secondary Antibody (Catalog #
F0107). View our protocol for Staining Membrane-associated Proteins.
Detection of Mouse Human/Mouse/Rat CD31/PECAM-1 Antibody by Immunohistochemistry
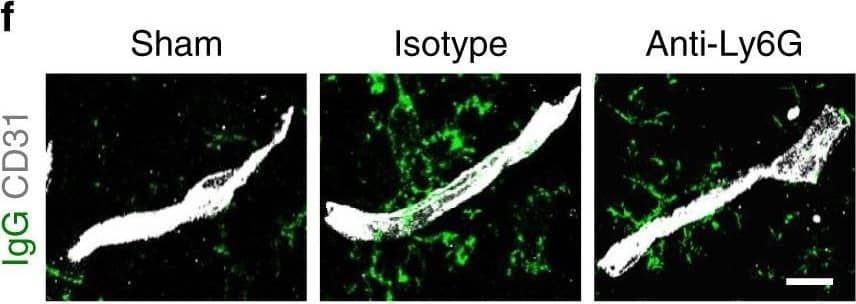
Neutrophil depletion reduces BBB breakdown and increases neovascularization after stroke. f, g Representative confocal images (f) and quantitative analysis of IgG extravascular deposits (g) in the peri-infarct cortex at 14 days in sham-operated mice and mice treated with control antibody or anti-Ly6G antibody (n = 6). One-way ANOVA test was applied with *P < 0.0001 (Sham vs. Isotype), *P = 0.0041 (Isotype vs. Anti-Ly6G). Bar = 15 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32427863), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Human/Mouse/Rat CD31/PECAM-1 Antibody by Immunohistochemistry
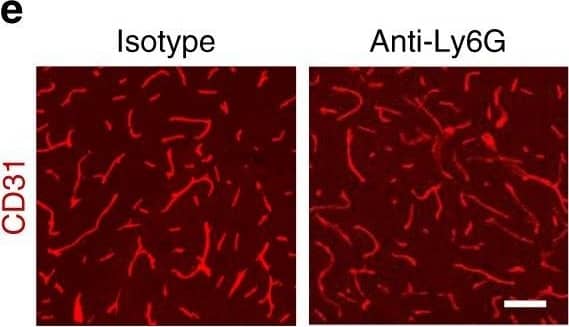
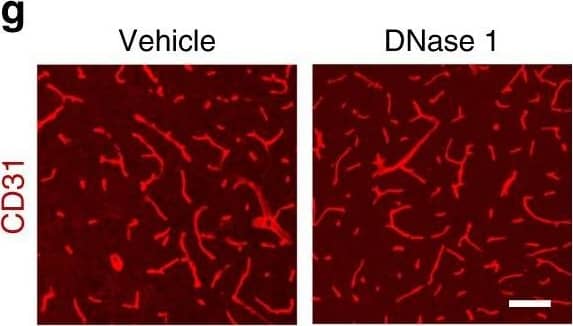
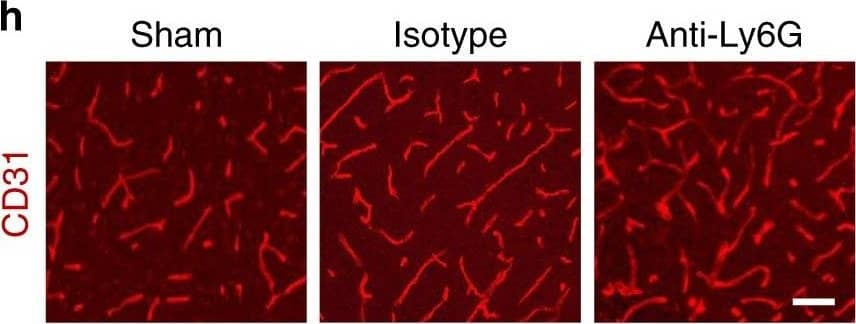
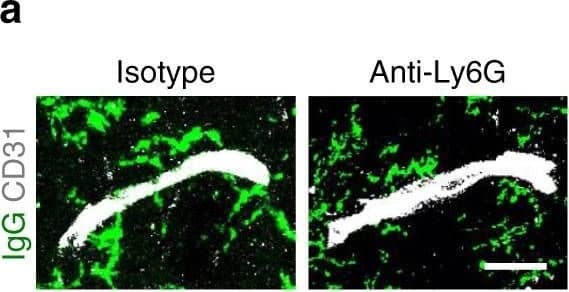
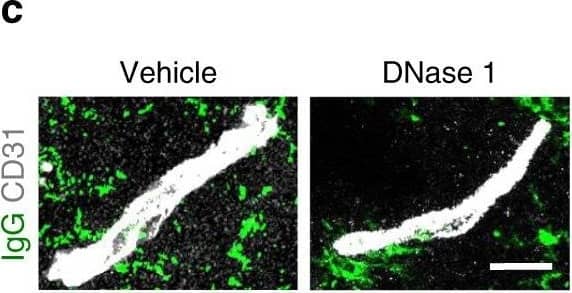
Increased vascular remodeling by delayed inhibition of NET formation.a–d Representative confocal images (a, c) and quantitative analysis of IgG extravascular deposits (b, d) in the peri-infarct cortex at 14 days. Mice were subjected to stroke and treated with either anti-Ly6G antibody, control antibody, DNase 1, or vehicle starting at 7 days (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.0392 (b), *P = 0.0384 (d). Bar = 10 μm. e–l Representative confocal images (e, g) of CD31-positive microvessels and in-vivo multiphoton microscopy images of perfused cortical capillaries with intravenously injected FITC-dextran (i, k) in the peri-infarct cortex at 14 days in mice treated with either anti-Ly6G antibody, control antibody, DNase 1, or vehicle. Bar = 40 μm (e, g) and 100 µm (i, k). Quantification of microvascular density (f, h) and perfused capillary length (j, l) for each group (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.00378 (f), *P = 0.0364 (h), *P = 0.0026 (j), *P = 0.0006 (l). Data are presented as mean ± SD. Source data underlying graph b, d, f, h, j, and l are provided as a Source Data file. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32427863), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Human/Mouse/Rat CD31/PECAM-1 Antibody by Immunohistochemistry
Increased vascular remodeling by delayed inhibition of NET formation.a–d Representative confocal images (a, c) and quantitative analysis of IgG extravascular deposits (b, d) in the peri-infarct cortex at 14 days. Mice were subjected to stroke and treated with either anti-Ly6G antibody, control antibody, DNase 1, or vehicle starting at 7 days (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.0392 (b), *P = 0.0384 (d). Bar = 10 μm. e–l Representative confocal images (e, g) of CD31-positive microvessels and in-vivo multiphoton microscopy images of perfused cortical capillaries with intravenously injected FITC-dextran (i, k) in the peri-infarct cortex at 14 days in mice treated with either anti-Ly6G antibody, control antibody, DNase 1, or vehicle. Bar = 40 μm (e, g) and 100 µm (i, k). Quantification of microvascular density (f, h) and perfused capillary length (j, l) for each group (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.00378 (f), *P = 0.0364 (h), *P = 0.0026 (j), *P = 0.0006 (l). Data are presented as mean ± SD. Source data underlying graph b, d, f, h, j, and l are provided as a Source Data file. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32427863), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Human/Mouse/Rat CD31/PECAM-1 Antibody by Immunohistochemistry
Neutrophil depletion reduces BBB breakdown and increases neovascularization after stroke. h, j Representative confocal images (h) of CD31-positive microvessels and in-vivo multiphoton microscopy images of perfused cortical capillaries with intravenously injected FITC-dextran (MW = 2000,000 Da) (j) in the peri-infarct cortex at 14 days in mice treated with control antibody or anti-Ly6G antibody, compared with sham-operated mice. Bar = 50 μm (e) and 100 µm (g) Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32427863), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Human/Mouse/Rat CD31/PECAM-1 Antibody by Immunohistochemistry
Increased vascular remodeling by delayed inhibition of NET formation.a–d Representative confocal images (a, c) and quantitative analysis of IgG extravascular deposits (b, d) in the peri-infarct cortex at 14 days. Mice were subjected to stroke and treated with either anti-Ly6G antibody, control antibody, DNase 1, or vehicle starting at 7 days (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.0392 (b), *P = 0.0384 (d). Bar = 10 μm. e–l Representative confocal images (e, g) of CD31-positive microvessels and in-vivo multiphoton microscopy images of perfused cortical capillaries with intravenously injected FITC-dextran (i, k) in the peri-infarct cortex at 14 days in mice treated with either anti-Ly6G antibody, control antibody, DNase 1, or vehicle. Bar = 40 μm (e, g) and 100 µm (i, k). Quantification of microvascular density (f, h) and perfused capillary length (j, l) for each group (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.00378 (f), *P = 0.0364 (h), *P = 0.0026 (j), *P = 0.0006 (l). Data are presented as mean ± SD. Source data underlying graph b, d, f, h, j, and l are provided as a Source Data file. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32427863), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Human/Mouse/Rat CD31/PECAM-1 Antibody by Immunohistochemistry
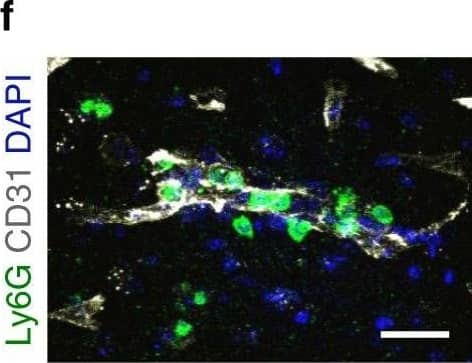
Neutrophils accumulate in the brain during all stages of ischemic stroke. f Representative confocal images of Ly6G-labeled neutrophils (green) and CD31-positive microvessels (white) in the peri-infarct cortex of mice at 3 days. Nuclei were visualized with Hoechst. Neutrophils were observed within brain vessels and migrated into the parenchyma. Bar = 40 μm. Independent experiments are repeated at least three times. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32427863), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Human/Mouse/Rat CD31/PECAM-1 Antibody by Immunohistochemistry
Diminished permeability of liver sinusoids in Plvap-deficient mice.Neither by immunohistochemistry with antibodies against CD31 (A) nor by light microscopy of 1 µm semi-thin sections (B, Richardson's stain) obvious differences are detected with regards to the overall orientation and the density of liver sinusoids between 3-week-old Plvap-/- mice and wild-type littermates. Sinusoids of Plvap-deficient mice show a higher number of macrophages in their lumen (white arrows) and focal areas with accumulations of mononuclear cells in Disse's space (black arrows). Lower panels in B show higher magnifications. C, After perfusion of a wild-type animal with FITC-dextran, a strong FITC-signal (green) throughout the liver is detected. Immunolabeling with CD31 (red) suggests that FITC-dextran molecules have accumulated in the space of Disse. In contrast, in the Plvap-deficient littermate, the signal for FITC-dextran is much weaker and barely detectable. Nuclear DNA is labeled with DAPI (blue). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25541982), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Human/Mouse/Rat CD31/PECAM-1 Antibody by Immunohistochemistry
Increased vascular remodeling by delayed inhibition of NET formation.a–d Representative confocal images (a, c) and quantitative analysis of IgG extravascular deposits (b, d) in the peri-infarct cortex at 14 days. Mice were subjected to stroke and treated with either anti-Ly6G antibody, control antibody, DNase 1, or vehicle starting at 7 days (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.0392 (b), *P = 0.0384 (d). Bar = 10 μm. e–l Representative confocal images (e, g) of CD31-positive microvessels and in-vivo multiphoton microscopy images of perfused cortical capillaries with intravenously injected FITC-dextran (i, k) in the peri-infarct cortex at 14 days in mice treated with either anti-Ly6G antibody, control antibody, DNase 1, or vehicle. Bar = 40 μm (e, g) and 100 µm (i, k). Quantification of microvascular density (f, h) and perfused capillary length (j, l) for each group (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.00378 (f), *P = 0.0364 (h), *P = 0.0026 (j), *P = 0.0006 (l). Data are presented as mean ± SD. Source data underlying graph b, d, f, h, j, and l are provided as a Source Data file. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32427863), licensed under a CC-BY license. Not internally tested by R&D Systems.
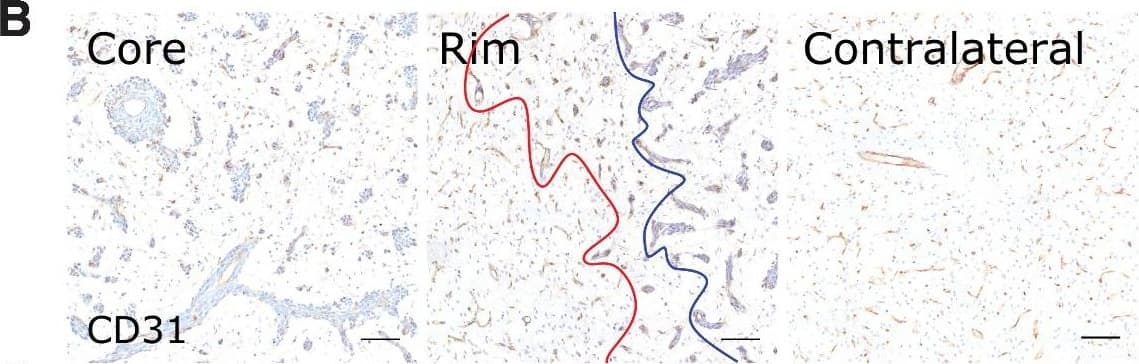
Detection of Mouse CD31/PECAM-1 by Immunohistochemistry
Microvascular and tumor cell biology at the tumor rim in MDA231Br-GFP brain metastases. Representative histologic sections of rat brains, intrastriatally injected with MDA231Br-GFP tumor cells, from the tumor core, tumor rim, and the contralateral striatum, with corresponding box and whisker plots of marker expression in each region. Data shown as median ± interquartile range. Sections were immunohistochemically stained (brown) for tumor cell marker vimentin (A), endothelial marker CD31 (B), cell adhesion molecule VCAM-1 (C), cell proliferation marker Ki67 (D), and two stemness markers: SOX2 (E) and nestin (F). Scale bar = 100 μm. *, P < 0.05; ***, P < 0.001; n = 8; post hoc Bonferroni multiple comparison test for tumor cell density and microvessel density, and post hoc Dunn test for VCAM-1 expression. Tumor core was delineated from the infiltrative border as indicated by the blue and red lines, respectively. Expression of Ki67, nestin and SOX2 has been normalized to tumor area. *, P < 0.05; ***, P < 0.001; matched Wilcoxon test. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35312755), licensed under a CC-BY license. Not internally tested by R&D Systems.
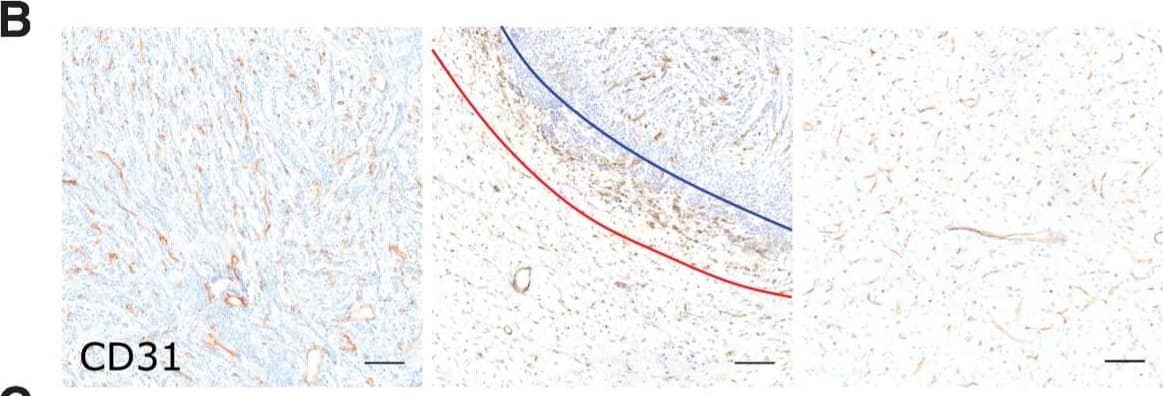
Detection of Human CD31/PECAM-1 by Immunohistochemistry
Microvascular and tumor cell biology at the tumor rim in U87MG glioblastoma. Representative histologic sections of rat brains, intrastriatally injected with U87MG tumor cells, from the tumor core, tumor rim, and the contralateral striatum, with corresponding box and whisker plots of marker expression in each region. Data shown as median ± interquartile range. Sections were immunohistochemically stained (brown) for tumor cell marker vimentin (A), endothelial marker CD31 (B), cell adhesion molecule VCAM-1 (C), cell proliferation marker Ki67 (D), and two stemness markers: SOX2 (E) and nestin (F). Scale bar = 100 μm. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n = 5; post hoc Bonferroni multiple comparison test for tumor cell density and microvessel density, and post hoc Dunn test for VCAM-1 expression. Tumor core was delineated from the infiltrative border as indicated by the blue and red lines, respectively. Expression of Ki67, nestin, and SOX2 has been normalized to tumor area. **, P < 0.01; matched Wilcoxon test. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35312755), licensed under a CC-BY license. Not internally tested by R&D Systems.