Human AGTR-2 PE-conjugated Antibody
R&D Systems, part of Bio-Techne | Catalog # FAB3659P

Conjugate
Catalog #
Key Product Details
Species Reactivity
Human
Applications
Flow Cytometry
Label
Phycoerythrin (Excitation = 488 nm, Emission = 565-605 nm)
Antibody Source
Monoclonal Mouse IgG2B Clone # 364805
Product Specifications
Immunogen
HEK293 human embryonic kidney cell line transfected with human AGTR-2
Met1-Ser363
Accession # P50052
Met1-Ser363
Accession # P50052
Specificity
Detects human AGTR‑2. Stains human AGTR-2-transfected cells but not irrelevant transfectants.
Clonality
Monoclonal
Host
Mouse
Isotype
IgG2B
Scientific Data Images for Human AGTR-2 PE-conjugated Antibody
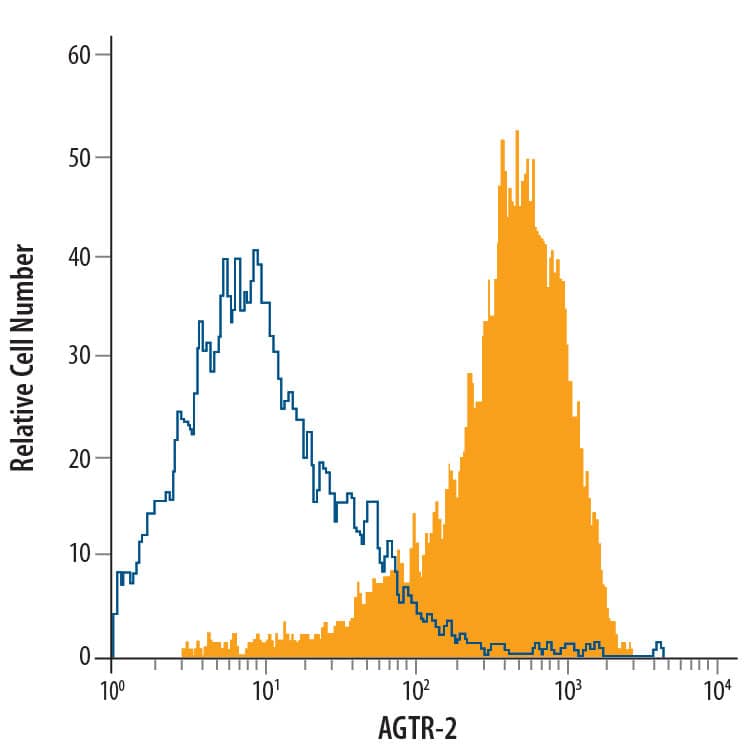
Detection of AGTR-2 in NS0 Mouse Cell Line Transfected with AGTR-2 by Flow Cytometry.
NS0 mouse myeloma cell line transfected with AGTR-2 was stained with Mouse Anti-Human AGTR-2 PE-conjugated Monoclonal Antibody (Catalog # FAB3659P, filled histogram) or isotype control antibody (Catalog # IC0041P, open histogram). View our protocol for Staining Membrane-associated Proteins.Applications for Human AGTR-2 PE-conjugated Antibody
Application
Recommended Usage
Flow Cytometry
10 µL/106 cells
Sample: NS0 mouse myeloma cell line transfected with AGTR-2
Sample: NS0 mouse myeloma cell line transfected with AGTR-2
Formulation, Preparation, and Storage
Purification
Protein A or G purified from hybridoma culture supernatant
Formulation
Supplied in a saline solution containing BSA and Sodium Azide.
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below.
Stability & Storage
Protect from light. Do not freeze.
- 12 months from date of receipt, 2 to 8 °C as supplied.
Background: AGTR-2
Long Name
Angiostensin II Type 2 Receptor
Alternate Names
AGTR2, AT2, AT2R, MRX88
Gene Symbol
AGTR2
UniProt
Additional AGTR-2 Products
Product Documents for Human AGTR-2 PE-conjugated Antibody
Product Specific Notices for Human AGTR-2 PE-conjugated Antibody
For research use only
Loading...
Loading...
Loading...
Loading...
Loading...