Human CD23/Fc epsilon RII PE-conjugated Antibody
R&D Systems, part of Bio-Techne | Catalog # FAB123P

Key Product Details
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
Met150-Ser321
Accession # P06734
Specificity
Clonality
Host
Isotype
Scientific Data Images for Human CD23/Fc epsilon RII PE-conjugated Antibody
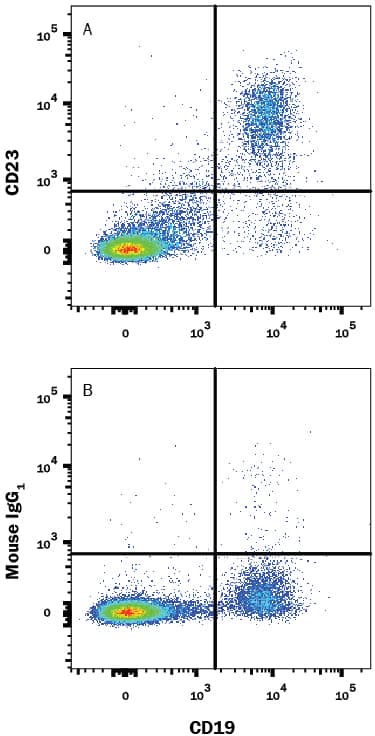
Detection of CD23/Fc epsilon RII in Human Blood Lymphocytes by Flow Cytometry.
Human peripheral blood lymphocytes were stained with Mouse Anti-Human CD19 APC-conjugated Monoclonal Antibody (Catalog # FAB4867A) and either (A) Mouse Anti-Human CD23/Fce RII PE-conjugated Monoclonal Antibody (Catalog # FAB123P) or (B) Mouse IgG1Phycoerythrin Isotype Control (Catalog # IC002P). View our protocol for Staining Membrane-associated Proteins.Applications for Human CD23/Fc epsilon RII PE-conjugated Antibody
Flow Cytometry
Sample: Human peripheral blood lymphocytes
Formulation, Preparation, and Storage
Purification
Formulation
Shipping
Stability & Storage
Background: CD23/Fc epsilon RII
CD23 (also named B cell differentiation antigen) is a member of subgroup II of the C-type (Ca++-dependent) lectin superfamily (1‑5). Human CD23 is a 47 kDa type II transmembrane glycoprotein that is expressed by a wide variety of cell types (6‑10). The full-length receptor is 321 amino acids (aa) in length and contains a 274 aa extracellular region, a 26 aa transmembrane segment, and a 21 aa cytoplasmic domain. The extracellular region contains a C-type lectin domain and a connecting stalk with coiled-coil topography (3, 11). The lectin domain binds both protein and carbohydrate in an apparently Ca++ independent manner (11). The coiled-coil region contributes to oligomerization (11, 12). The lectin domain in human CD23 (aa 162‑284) is 64%, 62% and 68% aa identical to the lectin domains in mouse, rat and bovine CD23, respectively. In the cytoplasmic region, two FC isoforms exist which arise from alternate start sites (6, 12). The “a” (or long) isoform begins with the sequence MEEGQYS and is constitutively expressed by B cells. It is believed to participate in IgE-mediated endocytosis (13). The “b” (or short) isoform begins with MNPPSQ and is induced on a wide variety of cell types by IL-4 (6). Fcb reportedly contributes to IgE-mediated phagocytosis (13). Fcb expressing cells include eosinophils, monocytes, visceral smooth muscle and intestinal epithelium (6, 14, 15). At least four soluble forms of CD23 are known to exist. They range in molecular weight from 25 kDa to 37 kDa, with the 25 kDa form predominating in sera (16). Soluble CD23 (sFc) is generated by metalloprotease (ADAM8; ADAM15; ADAM28) and cysteine-protease activity (16‑18). Cleavage usually occurs between aa 150‑160 (7, 8). It is unclear if sequential metalloprotease-cysteine protease activity is necessary for the generation of all soluble forms. Both soluble and membrane-bound CD23 show bioactivity. Ligands for CD23 include CD21, IgE, CD11b, and CD11c (19‑21). CD23 binding to CD11b and Cd11c on monocytes results in oxidative product generation and proinflammatory cytokine release (21). On B cells, sCD23 induces IgE secretion by binding CD21. Conversely, secreted IgE will, in turn, bind B cell membrane CD23, rendering it unavailable for cleavage, and thus shutting down IgE production (11).
References
- Kijimoto-Ochiai, S. (2002) Cell. Mol. Life Sci. 59:648.
- Heyman, B. (2000) Annu. Rev. Immunol. 18:709.
- Bajorath, J. and A. Aruffo (1996) Protein Sci. 5:240.
- Drickamer, K. (1993) Curr. Opin. Struct. Biol. 3:393.
- Drickamer, K. (1999) Curr. Opin. Struct. Biol. 9:585.
- Yokota, A. et al. (1988) Cell 55:611.
- Ludin, C. et al. (1987) EMBO J. 6:109.
- Ikuta, K. et al. (1987) Proc. Natl. Acad. Sci. USA 84:819.
- Kikutani, H. et al. (1986) Cell 47:657.
- Letellier, M. et al. (1988) J. Immunol. 141:2374.
- Hibbert, R.G. et al. (2005) J. Exp. Med. 202:751.
- Beavuil, A.J. et al. (1992) Proc. Natl. Acad. Sci. USA 89:753.
- Yokota, A. et al. (1992) Proc. Natl. Acad. Sci. USA 89:5030.
- Belleau, J.T. et al. (2005) Clin. Mol. Allergy 3:6.
- Tu, Y. et al. (2005) Gastroenterology 129:928.
- Marolewski, A.E. et al. (1998) Biochem. J. 333:573.
- Fourie, A.M. et al. (2003) J. Biol. Chem. 278:30469.
- Karagiannis, S.N. et al. (2001) Immunology 103:319.
- Aubry, J-P. et al. (1992) Nature 358:505.
- Sarfati, M. and G. Delespeese (1988) J. Immunol. 141:2195.
- Lecoanet-Henchoz, S. et al. (1995) Immunity 3:119.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional CD23/Fc epsilon RII Products
Product Documents for Human CD23/Fc epsilon RII PE-conjugated Antibody
Product Specific Notices for Human CD23/Fc epsilon RII PE-conjugated Antibody
For research use only