Human HAPLN1 Antibody
R&D Systems, part of Bio-Techne | Catalog # AF2608

Key Product Details
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
Asp16-Asn354
Accession # P10915
Specificity
Clonality
Host
Isotype
Scientific Data Images for Human HAPLN1 Antibody
Detection of Human HAPLN1 by Immunocytochemistry/Immunofluorescence
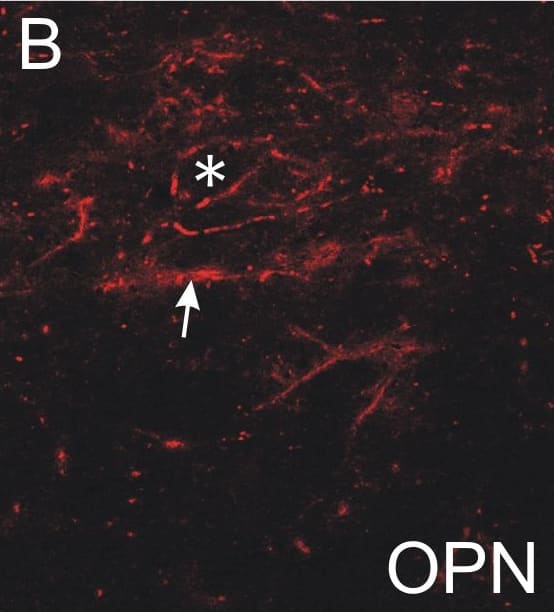
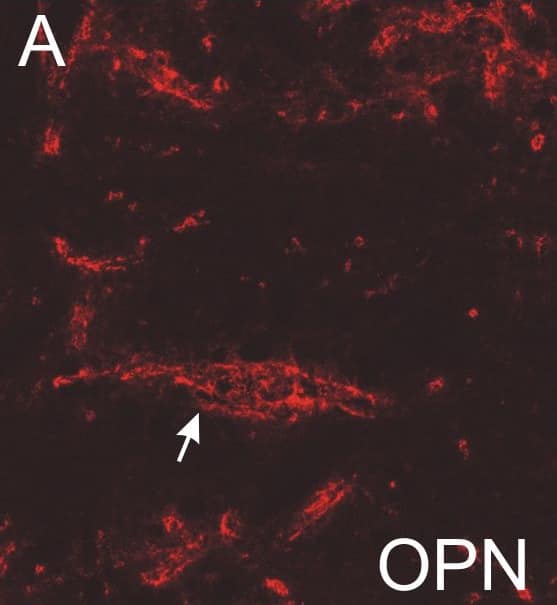
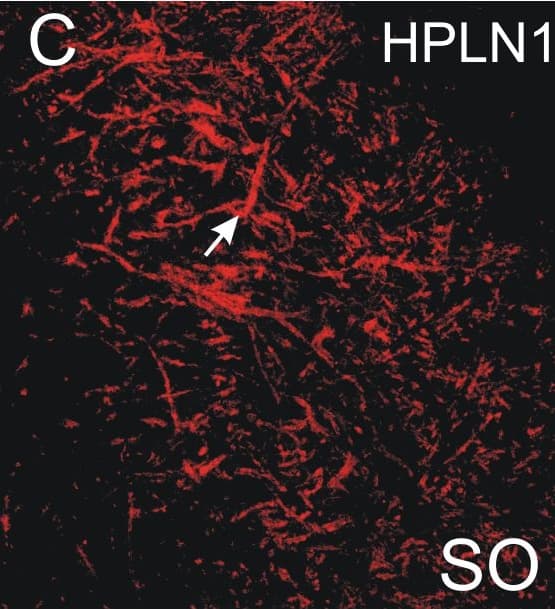
Absent or fragmented omnipause neuron perineuronal net triple immunofluorescence staining.Triple immunofluorescence staining for different components of perineuronal nets, revealed by a confocal laser scanning microscope. In the control case, omnipause neurons (OPN) are ensheathed by prominent perineuronal nets showing the same appearance with antibodies against the link protein (HPLN1), chondroitin sulfate proteoglycan (CSPG) and aggrecan (ACAN) (A, D, G, arrow). In the saccadic palsy patient, the neurons of the superior olive (SO) from the same sections as OPN are ensheathed by prominent perineuronal nets revealed by immunostaining of HPLN1, CSPG, and ACAN (C, F, I, arrows). However, around OPN (asterisk) in the patient, only HPLN1-based perineuronal nets can be detected, which appear fragmented (B, arrow). CSPG- and ACAN-immunostaining does not reveal perineuronal nets, but only few fragments along a few dendrites (E, H, arrow). Scale bars A,D,G = 20μm; B,C,E,F,H,I = 200μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26135580), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of Human HAPLN1 by Immunocytochemistry/Immunofluorescence
Absent or fragmented omnipause neuron perineuronal net triple immunofluorescence staining.Triple immunofluorescence staining for different components of perineuronal nets, revealed by a confocal laser scanning microscope. In the control case, omnipause neurons (OPN) are ensheathed by prominent perineuronal nets showing the same appearance with antibodies against the link protein (HPLN1), chondroitin sulfate proteoglycan (CSPG) and aggrecan (ACAN) (A, D, G, arrow). In the saccadic palsy patient, the neurons of the superior olive (SO) from the same sections as OPN are ensheathed by prominent perineuronal nets revealed by immunostaining of HPLN1, CSPG, and ACAN (C, F, I, arrows). However, around OPN (asterisk) in the patient, only HPLN1-based perineuronal nets can be detected, which appear fragmented (B, arrow). CSPG- and ACAN-immunostaining does not reveal perineuronal nets, but only few fragments along a few dendrites (E, H, arrow). Scale bars A,D,G = 20μm; B,C,E,F,H,I = 200μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26135580), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of Human HAPLN1 by Immunocytochemistry/Immunofluorescence
Absent or fragmented omnipause neuron perineuronal net triple immunofluorescence staining.Triple immunofluorescence staining for different components of perineuronal nets, revealed by a confocal laser scanning microscope. In the control case, omnipause neurons (OPN) are ensheathed by prominent perineuronal nets showing the same appearance with antibodies against the link protein (HPLN1), chondroitin sulfate proteoglycan (CSPG) and aggrecan (ACAN) (A, D, G, arrow). In the saccadic palsy patient, the neurons of the superior olive (SO) from the same sections as OPN are ensheathed by prominent perineuronal nets revealed by immunostaining of HPLN1, CSPG, and ACAN (C, F, I, arrows). However, around OPN (asterisk) in the patient, only HPLN1-based perineuronal nets can be detected, which appear fragmented (B, arrow). CSPG- and ACAN-immunostaining does not reveal perineuronal nets, but only few fragments along a few dendrites (E, H, arrow). Scale bars A,D,G = 20μm; B,C,E,F,H,I = 200μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26135580), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Human HAPLN1 Antibody
Western Blot
Sample: Recombinant Human HAPLN1 (Catalog # 2608-HP)
Reviewed Applications
Read 2 reviews rated 5 using AF2608 in the following applications:
Formulation, Preparation, and Storage
Purification
Reconstitution
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: HAPLN1
HAPLN1 (also known as link protein and CRTL1) is a member of the hyaladherin family of hyaluronic acid (HA) binding proteins. Hyaluronan binding proteins are of two types; those with link modules, and those without. Link modules are 100 amino acid (aa) HA and protein-binding sequences that contain two alpha-helices and two antiparallel beta-sheets (1, 3). There are three categories of link module-containing proteins. “A” domain-type proteins contain one link module; “B” domain-type proteins contain one link module with an N- and C-terminal flanking region; and “C” domain-type proteins have an extended structure with one N-terminal V-type Ig-like domain followed by two link modules (2). The HAPLN family is a group of four C domain-type proteins that share approximately 50% aa identity (4). HAPLN1 is synthesized as a 354 aa precursor that contains a 15 aa signal sequence and a 339 aa mature region (4 - 6). It contains one Ig-like domain and two 95 aa link modules (6). It is variably glycosylated with a native molecular weight between 41 - 48 kDa (7, 8). Mature human HAPLN1 is 97%, 96%, 96%, 96%, and 96% aa identical to equine, porcine, rat, mouse and bovine HAPLN1, respectively. HAPLN1 contributes to extracellular matrix stability and flexibility (9). In cartilage, HALPN1 forms a ternary complex with HA and aggrecan. This creates a gel-like substance with remarkable resistance to deformation (3). In this complex, HA forms a linear backbone with perpendicularly attached aggrecan and HAPLN1. Aggrecan and HAPLN1 lie parallel to each other, while HA runs between the two HAPLN1 link modules (2, 3, 10). The Ig domain of HAPLN1 binds to aggrecan, while the two link modules of HAPLN1 bind to HA. Although HA and aggrecan will associate, the tendency is towards dissociation (2, 3, 8). HAPLN1 provides a stabilizing influence on HA-aggrecan associations, thus creating a long-lived ternary functional complex.
References
- Day, A.J. and G.D. Prestwich (2002) J. Biol. Chem. 277:4585.
- Seyfried, N.T. et al. (2005) J. Biol. Chem. 280:5435.
- Matsumoto, K. et al. (2003) J. Biol. Chem. 278:41205.
- Spicer, A.P. et al. (2003) J. Biol. Chem. 278:21083.
- Dudhia, J. and T.E. Hardingham (1990) Nucleic Acids Res. 18:1292.
- Osborne-Lawrence, S.L. et al. (1990) Genomics 8:562.
- Roughley, P.J. et al. (1982) J. Biol. Chem. 257:11908.
- Shi, S. et al. (2004) J. Biol. Chem. 279:12060.
- Binette, F. et al. (1994) J. Biol. Chem. 269:19116.
- Perkins, S.J. et al. (1992) Biochem. J. 285:263.
Long Name
Alternate Names
Entrez Gene IDs
Gene Symbol
UniProt
Additional HAPLN1 Products
Product Documents for Human HAPLN1 Antibody
Product Specific Notices for Human HAPLN1 Antibody
For research use only