Human Serpin A10/ZPI Antibody
R&D Systems, part of Bio-Techne | Catalog # MAB8115

Key Product Details
Species Reactivity
Applications
Label
Antibody Source
Product Specifications
Immunogen
Met1-Leu444
Accession # Q9UK55
Specificity
Clonality
Host
Isotype
Scientific Data Images for Human Serpin A10/ZPI Antibody
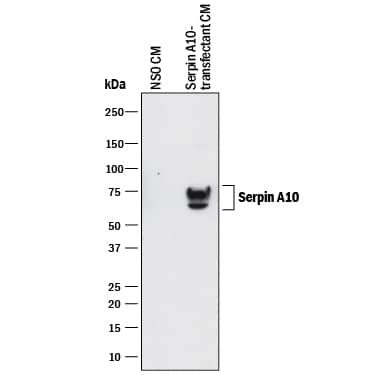
Detection of Human Serpin A10/ZPI by Western Blot.
Western blot shows conditioned media of NS0 mouse myeloma cell line either mock transfected or transfected with human Serpin A10. PVDF membrane was probed with 1 µg/mL of Mouse Anti-Human Serpin A10/ZPI Monoclonal Antibody (Catalog # MAB8115) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF018). Specific bands were detected for Serpin A10/ ZPI at approximately 70-75 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.Applications for Human Serpin A10/ZPI Antibody
Western Blot
Sample: NS0 mouse myeloma cell line transfected with human Serpin A10
Formulation, Preparation, and Storage
Purification
Reconstitution
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Serpin A10/ZPI
Protein Z-dependent Protease Inhibitor (ZPI), also known as SerpinA10 (SERine Proteinase INhibitor-clade A10) is a monomeric, secreted member of the A (or extracellular) clade within the serpin superfamily of protease inhibitors (1-4). In general, members of this superfamily regulate multiple proteolytic cascades, and are particularly effective due to the fact that their inhibitory activities can be fine-tuned through the participation of discrete, non-serpin co-factors (4). Serpins are unusual in that they are one-time use, non-recyclable proteins whose native state is thermodynamically unstable. They act as non-physiologic substrates for enzymes that, once cleaved, form a covalent bond with the target enzyme, rendering it inactive (1, 2). ZPI is a hepatocyte-derived glycoprotein associated with the coagulation cascade (3, 5-7). Following vessel damage, underlying support collagen and fibroblasts are exposed to circulating plasma and platelets. This results in the activation of two coagulation pathways; an intrinsic pathway involving platelets, and an extrinsic pathway involving vascular stromal cells. Both pathways converge at the activation step for factor X, which converts prothrombin into thrombin, a prelude to the generation of fibrin. ZPI negatively regulates the activation state of two coagulation factor enzymes; factor XIa and factor Xa (5-7). Factor XI is unique to the intrinsic pathway, while factor X, as noted, is common to both pathways. Inhibition is most efficiently accomplished by ZPI binding to either factor Xa (with protein Z [PZ], calcium and phospholipids) or XIa (lacking non-heparin co-factors), precluding them from activating downstream zymogens. Binding is accompanied by serpin cleavage, but unlike a typical serpin, ZPI does not stay bound to enzyme; rather, it dissociates into a 4 kDa C-terminal fragment and a 68 kDa N-terminal sequence (6, 8, 9). Following dissociation, a second ZPI molecule is recruited, and the process repeated. In humans, the majority of ZPI circulates in a complex with PZ. PZ serves as an intermediary, bringing ZPI in contact with factor Xa or XIa on the surface of platelets (1, 10-12). Once cleaved, ZPI dissociates from PZ, and PZ is free to bind and present additional ZPI to Xa and XIa. Cell-surface heparan sulfate on endothelium is also reported to act as a scaffold for ZPI:Xa interactions (8, 13). Human and mouse systems are not strictly analogous, as ZPI > PZ in human blood, while PZ>ZPI in mouse blood (11). Mature human ZPI is 423 amino acids (aa) in length (aa 22-444), and it contains a factor Xa cleavage site between Tyr408Ser409. Human and mouse ZPI share 71% aa sequence identity. Notably on SDS-PAGE, human ZPI runs about 72 kDa, while mouse and rat ZPI exhibit MWs of 91 kDa and 51 kDa, respectively (14).
References
- Chen, H. et al. (2013) Cardiovasc. Haematol. Disord. Drug Targets 13:99.
- Law, R.H.P. et al. (2006) Genome Biol. 7:216.
- Han, X. et al. (1999) Biochemistry 38:11073.
- Corral, J. et al. (2007) Br. J. Haematol. 137:99.
- Han, X. et al. (1998) Proc. Natl. Acad. Sci. USA 95:9250.
- Han, X. et al. (2000) Blood 96:3049.
- Broze, G.J. et al. (2001) Thromb. Haemost. 86:8.
- Vasse, M. (2011) Hamostaseologie 31:155.
- Huang, X. et al. (2012) Blood 120:1726.
- Huang, X. et al. (2010) J. Biol. Chem. 285:20399.
- Girard, T.J. et al. (2013) J. Thromb. Haemost. 11:375.
- Wei, Z. et al. (2009) Blood 114:3662.
- Huang, X. et al. (2011) J. Biol. Chem. 286:8740.
- Zhang, J. & G.J. Broze (2001) Thromb. Haemost. 85:861.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional Serpin A10/ZPI Products
Product Documents for Human Serpin A10/ZPI Antibody
Product Specific Notices for Human Serpin A10/ZPI Antibody
For research use only