Mouse Follistatin-like 1/FSTL1 Antibody
R&D Systems, part of Bio-Techne | Catalog # AF1738

Key Product Details
Validated by
Biological Validation
Species Reactivity
Validated:
Mouse
Cited:
Mouse, Rat
Applications
Validated:
Western Blot
Cited:
Immunocytochemistry, Immunohistochemistry, Immunohistochemistry-Frozen, Immunohistochemistry-Paraffin, Immunoprecipitation, Western Blot
Label
Unconjugated
Antibody Source
Polyclonal Goat IgG
Product Specifications
Immunogen
Mouse myeloma cell line NS0-derived recombinant mouse Follistatin-like 1/FSTL1
Glu19-Ile306
Accession # Q62356
Glu19-Ile306
Accession # Q62356
Specificity
Detects mouse Follistatin-like 1/FSTL1 in direct ELISAs and Western blots. In Western blots, approximately 5% cross-reactivity with recombinant human Follistatin-like 1/FSTL1 is observed.
Clonality
Polyclonal
Host
Goat
Isotype
IgG
Scientific Data Images for Mouse Follistatin-like 1/FSTL1 Antibody
Detection of Mouse Follistatin-like 1/FSTL1 by Immunohistochemistry
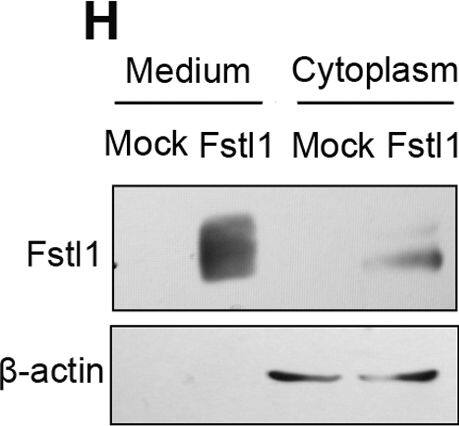
Proliferation defects in Fstl1-/- ureteric epithelial cells.(A-D) Analysis of cell proliferation on transverse sections of wild-type (A, C) and Fstl1-/- (B, D) ureters by BrdU incorporation at E15.5 (A, B) and E16.5 (distal segment) (C, D). Arrows point to representative proliferating cells. (E) Quantification of cell proliferation by BrdU labeling index. In E15.5 ureter, p<0.001 for epithelial layer (n = 20) and p = 0.49 for mesenchymal inner layer (n = 20). In distal segment of E16.5 ureter, p<0.001 for epithelial layer (n = 20) and p = 0.59 for mesenchymal inner layer (n = 20). (F, G) BrdU incorporation analysis of epithelial cell proliferation on transverse section of cultured E15.0 ureters treated with conditioned media transfected either with pcDNA3.1 vector (Mock) (F) or an Fstl1 overexpress plasmid (G). Arrows point to representative proliferating cells. (H) HEK293 cells were transfected with a plasmid expressing mouse Fstl1 protein or pcDNA3.1 (mock). Western blot analysis using an anti-Fstl1 antibody indicated that Fstl1 was overexpressed in both the cultured media and cell pellet. (I) Quantification of cell proliferation by BrdU labeling index in the cultured E15.0 ureteric epithelium. p<0.005 (n = 7). Scale bar: (A-D, F-G) 20µm. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0032554), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of Human Follistatin-like 1/FSTL1 by Western Blot
Proliferation defects in Fstl1-/- ureteric epithelial cells.(A-D) Analysis of cell proliferation on transverse sections of wild-type (A, C) and Fstl1-/- (B, D) ureters by BrdU incorporation at E15.5 (A, B) and E16.5 (distal segment) (C, D). Arrows point to representative proliferating cells. (E) Quantification of cell proliferation by BrdU labeling index. In E15.5 ureter, p<0.001 for epithelial layer (n = 20) and p = 0.49 for mesenchymal inner layer (n = 20). In distal segment of E16.5 ureter, p<0.001 for epithelial layer (n = 20) and p = 0.59 for mesenchymal inner layer (n = 20). (F, G) BrdU incorporation analysis of epithelial cell proliferation on transverse section of cultured E15.0 ureters treated with conditioned media transfected either with pcDNA3.1 vector (Mock) (F) or an Fstl1 overexpress plasmid (G). Arrows point to representative proliferating cells. (H) HEK293 cells were transfected with a plasmid expressing mouse Fstl1 protein or pcDNA3.1 (mock). Western blot analysis using an anti-Fstl1 antibody indicated that Fstl1 was overexpressed in both the cultured media and cell pellet. (I) Quantification of cell proliferation by BrdU labeling index in the cultured E15.0 ureteric epithelium. p<0.005 (n = 7). Scale bar: (A-D, F-G) 20µm. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0032554), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of Mouse Follistatin-like 1/FSTL1 by Knockdown Validated
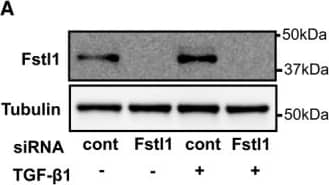
Endogenous Fstl1 does not contribute to TGF‐ beta 1/Smad 2/3 signaling or myofibroblast transdifferentiationFstl1 ablation by siRNA Fstl1 (6 pmol) in NRCFbs. siRNA Fstl1 or siRNA non‐targeting negative control was transfected to NRCFbs by lipofectamine RNAimax for 12 h, followed by stimulation of recombinant TGF‐ beta1 (10 ng/ml) for 24 h. Fstl1 protein expression in cell lysate was analyzed by immunoblotting.Efficiency of Fstl1 gene ablation by siRNA Fstl1 (6 pmol) in NRCFbs. siRNA Fstl1 or siRNA non‐targeting negative control was transfected to NRCFbs by lipofectamine RNAimax for 42 h. mRNA transcripts of Fstl1 and GAPDH were assessed by qPCR. The amplification plots for Fstl1 are shown. The Ct values for Fstl1 in siRNA control and siRNA Fstl1 are 18.17 ± 0.053 and 24.76 ± 0.420, respectively (n = 3 for each sample group).Effect of endogenous Fstl1 protein on TGF‐ beta1‐induced Smad2/3 signaling pathway in NRCFbs. Endogenous Fstl1 protein was ablated by transfecting siRNA Fstl1 to NRCFbs. siRNA negative control was used for control. Following FBS starvation for 24 h, cells were stimulated with recombinant TGF‐ beta1 protein (2 ng/ml) for 15 min. Phosphorylation of Smad2 and Smad3 was assessed by immunoblotting. Error bars represent mean ± SEM (n = 3 for each group). Statistical analysis was performed by ordinary one‐way ANOVA. Post hoc test was performed by Dunnett's T3 test for Smad2 and Tukey's test for Smad3. Two independent experiments were performed.Ablation of endogenous Fstl1 does not affect markers of myofibroblast differentiation or ECM protein synthesis. Endogenous Fstl1 was ablated by siRNA, and following FBS depletion for 24 h, cells were stimulated with recombinant Fstl1 protein (50 ng/ml) for 24 h. alpha‐SMA protein expression in cell lysate and fibronectin and collagen I protein in the cultured media were assessed by immunoblotting. Tubulin was used as an internal control. Error bars represent mean ± SEM (n = 4 for each group). Statistical analysis was performed by ordinary one‐way ANOVA and Tukey's test. Two independent experiments were performed.Source data are available online for this figure. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27234440), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Mouse Follistatin-like 1/FSTL1 Antibody
Application
Recommended Usage
Western Blot
0.1 µg/mL
Sample: Recombinant Mouse Follistatin-like 1/FSTL1
Sample: Recombinant Mouse Follistatin-like 1/FSTL1
Formulation, Preparation, and Storage
Purification
Antigen Affinity-purified
Reconstitution
Reconstitute at 0.2 mg/mL in sterile PBS. For liquid material, refer to CoA for concentration.
Formulation
Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. *Small pack size (SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Shipping
Lyophilized product is shipped at ambient temperature. Liquid small pack size (-SP) is shipped with polar packs. Upon receipt, store immediately at the temperature recommended below.
Stability & Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Follistatin-like 1/FSTL1
Alternate Names
Flik, Follistatin-related Protein, FRP, FSL1, FSTL1, TSC-36
Gene Symbol
FSTL1
UniProt
Additional Follistatin-like 1/FSTL1 Products
Product Documents for Mouse Follistatin-like 1/FSTL1 Antibody
Product Specific Notices for Mouse Follistatin-like 1/FSTL1 Antibody
For research use only
Loading...
Loading...
Loading...
Loading...