Mouse WARP Antibody
R&D Systems, part of Bio-Techne | Catalog # AF4927

Key Product Details
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
Arg19-Pro415
Accession # Q8R2Z5
Specificity
Clonality
Host
Isotype
Scientific Data Images for Mouse WARP Antibody
Detection of Mouse WARP/VWA1 by Immunocytochemistry/Immunofluorescence
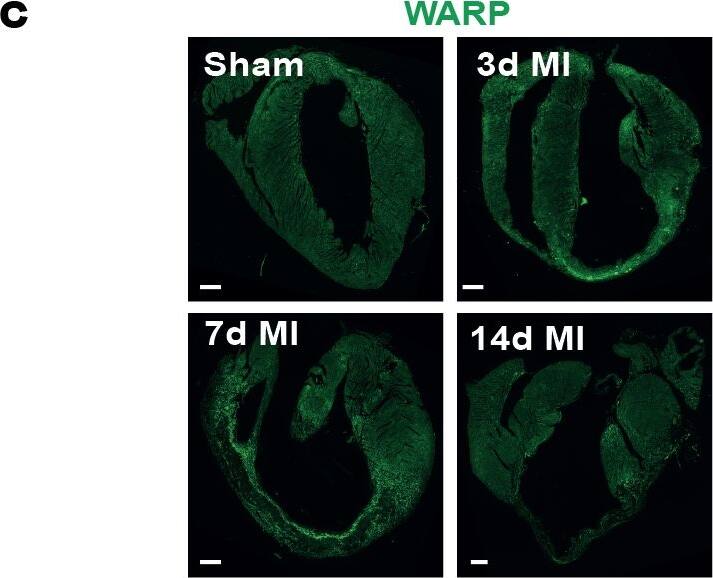
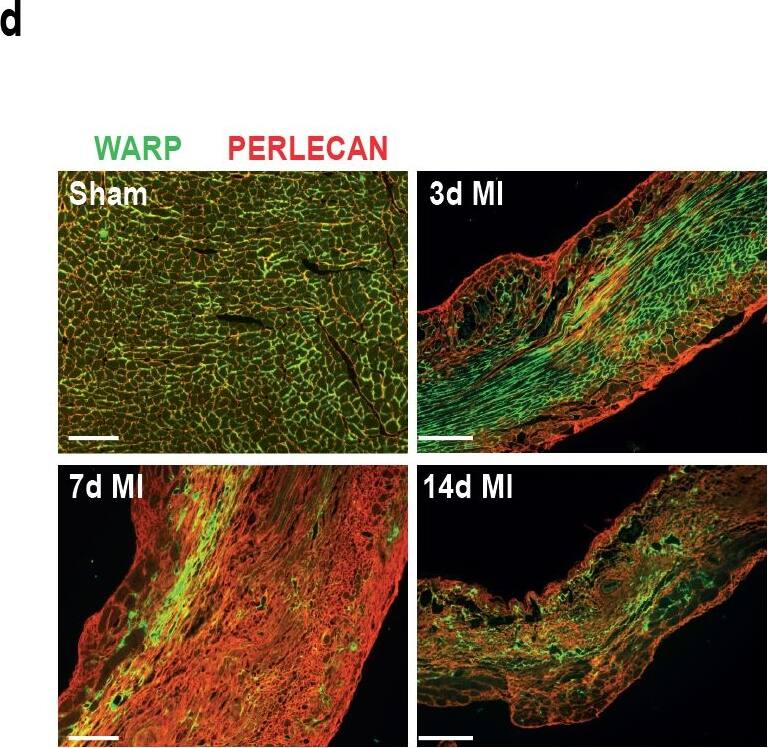
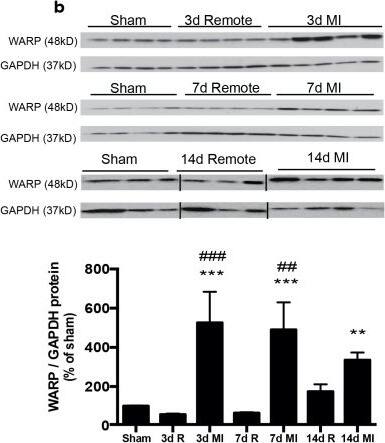
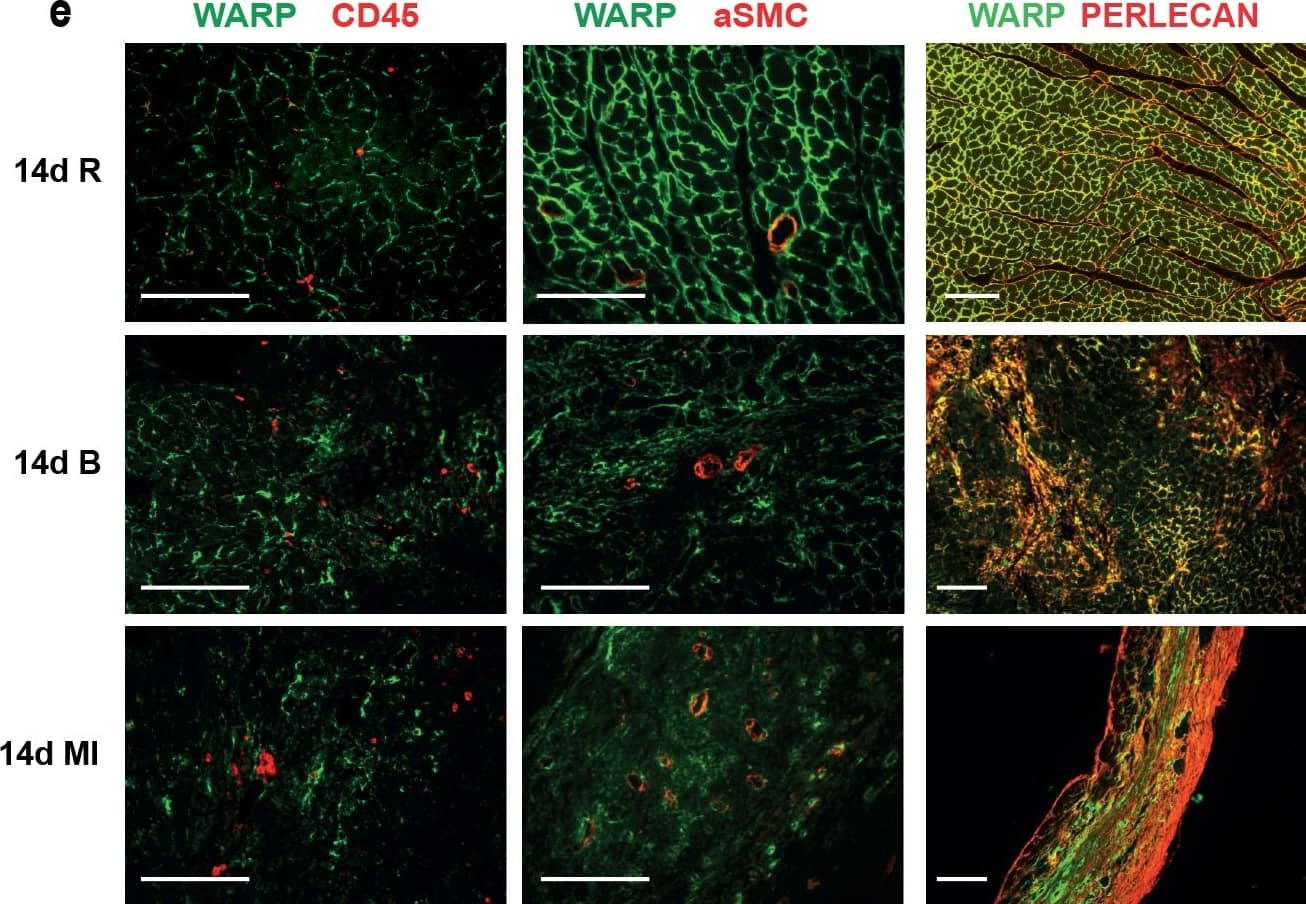
WARP expression pattern in the heart.(a) WARP mRNA levels were induced 3 and 7 days after MI. WARP mRNA levels peaked at 7 days after MI and then decreased again at 14 days. (b) Western blot analysis and (c) immunohistochemistry also showed an induction of WARP protein levels in the infarcted LV at 3, 7, and 14 days after MI. Because of different sample-loading, the blots of the 14 days MI were cut and pasted in the same order as the blots of the 3 and 7 days MI. Images of the original unadjusted blots are provided in S1 Fig (d and e) Confocal microscopy confirmed the co-localization of WARP with perlecan in the uninfarcted heart, showing a network of WARP and perlecan as a honeycomb-structure surrounding the cardiomyocytes. In the infarcted LV zones, the WARP-perlecan honeycomb-structure is reduced at 3 days after MI and completely absent at 7 and 14 days after MI and the interaction between WARP and perlecan is disrupted. (e) WARP did not localize on CD45 positive leukocytes or in alpha smooth muscle cells lining the vessels in the remote, border and infarcted zone of the heart 14 days after MI. n≥3; *p≤0.05; **p≤0.005; ***p≤0.001 versus sham, ##p≤0.005; ###p≤0.001 versus remote, bars 1000 μm for (c) and 100 μm for (d) and (e). Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0139199), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of Mouse WARP/VWA1 by Immunocytochemistry/Immunofluorescence
WARP expression pattern in the heart.(a) WARP mRNA levels were induced 3 and 7 days after MI. WARP mRNA levels peaked at 7 days after MI and then decreased again at 14 days. (b) Western blot analysis and (c) immunohistochemistry also showed an induction of WARP protein levels in the infarcted LV at 3, 7, and 14 days after MI. Because of different sample-loading, the blots of the 14 days MI were cut and pasted in the same order as the blots of the 3 and 7 days MI. Images of the original unadjusted blots are provided in S1 Fig (d and e) Confocal microscopy confirmed the co-localization of WARP with perlecan in the uninfarcted heart, showing a network of WARP and perlecan as a honeycomb-structure surrounding the cardiomyocytes. In the infarcted LV zones, the WARP-perlecan honeycomb-structure is reduced at 3 days after MI and completely absent at 7 and 14 days after MI and the interaction between WARP and perlecan is disrupted. (e) WARP did not localize on CD45 positive leukocytes or in alpha smooth muscle cells lining the vessels in the remote, border and infarcted zone of the heart 14 days after MI. n≥3; *p≤0.05; **p≤0.005; ***p≤0.001 versus sham, ##p≤0.005; ###p≤0.001 versus remote, bars 1000 μm for (c) and 100 μm for (d) and (e). Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0139199), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of Mouse WARP/VWA1 by Immunocytochemistry/Immunofluorescence
WARP expression pattern in the heart.(a) WARP mRNA levels were induced 3 and 7 days after MI. WARP mRNA levels peaked at 7 days after MI and then decreased again at 14 days. (b) Western blot analysis and (c) immunohistochemistry also showed an induction of WARP protein levels in the infarcted LV at 3, 7, and 14 days after MI. Because of different sample-loading, the blots of the 14 days MI were cut and pasted in the same order as the blots of the 3 and 7 days MI. Images of the original unadjusted blots are provided in S1 Fig (d and e) Confocal microscopy confirmed the co-localization of WARP with perlecan in the uninfarcted heart, showing a network of WARP and perlecan as a honeycomb-structure surrounding the cardiomyocytes. In the infarcted LV zones, the WARP-perlecan honeycomb-structure is reduced at 3 days after MI and completely absent at 7 and 14 days after MI and the interaction between WARP and perlecan is disrupted. (e) WARP did not localize on CD45 positive leukocytes or in alpha smooth muscle cells lining the vessels in the remote, border and infarcted zone of the heart 14 days after MI. n≥3; *p≤0.05; **p≤0.005; ***p≤0.001 versus sham, ##p≤0.005; ###p≤0.001 versus remote, bars 1000 μm for (c) and 100 μm for (d) and (e). Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0139199), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Mouse WARP Antibody
Western Blot
Sample: Recombinant Mouse WARP
Formulation, Preparation, and Storage
Purification
Reconstitution
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: WARP
WARP (von Willebrand factor A [vWFA] domain-related protein) is a 50 kDa glycoprotein member of the vWFA domain superfamily of extracellular matrix proteins. It is expressed in embryonic articular cartilage, skeletal muscle and basement membranes in the PNS. WARP forms disulfide-linked homodimers and multimers, and complexes with perlecan. Secreted mouse WARP is 397 amino acids (aa) in length. It contains a vWFA domain (aa 34‑209), and two fibronectin type III domains (aa 211‑394) that likely bind to the GAG modification of perlecan. Cys369 and Cys393 contribute to intermolecular bond formation. There is one alternate start site at Met213. Mature mouse WARP (aa 19‑415) is 93% and 78% aa identical to rat and human WARP, respectively.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional WARP Products
Product Documents for Mouse WARP Antibody
Product Specific Notices for Mouse WARP Antibody
For research use only