Complement Factor H-related 2/CFHR2/CFHL2 Antibody Pair [HRP]
Novus Biologicals, part of Bio-Techne | Catalog # NBP2-79419

Key Product Details
Assay Type
Sandwich ELISA
Assay Range
9.38-600 pg/ml (example only; lot dependent)
Sensitivity
9.38 pg/ml (example only; lot dependent)
Reactivity
Human
Product Specifications
Description
Solid Phase sandwich ELISA for the quantitative determination of Human Complement Factor H-related 2/CFHR2/CFHL2.
Sample Volume Required
100 ul
Conjugate
HRP
Scientific Data Images for Complement Factor H-related 2/CFHR2/CFHL2 Antibody Pair [HRP]
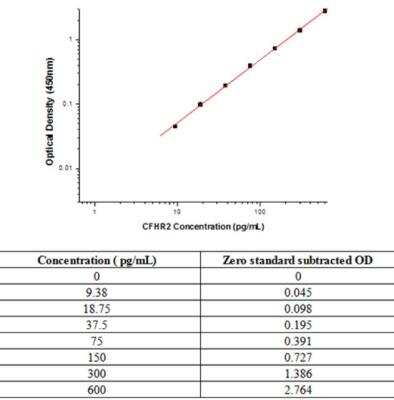
Sandwich ELISA: Complement Factor H-related 2/CFHR2/CFHL2 Antibody Pair [HRP] [NBP2-79419] - This standard curve is only for demonstration purposes. A standard curve should be generated for each assay.
Kit Contents for Complement Factor H-related 2/CFHR2/CFHL2 Antibody Pair [HRP]
- Mouse Monoclonal Capture Antibody
- Mouse Monoclonal Detection Antibody (HRP-conjugated)
- Standard
Preparation and Storage
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below.
Stability & Storage
Storage of components varies. See protocol for specific instructions.
Background: Complement Factor H-related 2/CFHR2
Alternate Names
CFHL2, CFHR2, Complement Factor Hrelated 2, FHR2, HFL3
Gene Symbol
CFHR2
Additional Complement Factor H-related 2/CFHR2 Products
- All Products for Complement Factor H-related 2/CFHR2
- Complement Factor H-related 2/CFHR2 cDNA Clones
- Complement Factor H-related 2/CFHR2 ELISA Kits
- Complement Factor H-related 2/CFHR2 Lysates
- Complement Factor H-related 2/CFHR2 Primary Antibodies
- Complement Factor H-related 2/CFHR2 Proteins and Enzymes
Product Documents for Complement Factor H-related 2/CFHR2/CFHL2 Antibody Pair [HRP]
Product Specific Notices for Complement Factor H-related 2/CFHR2/CFHL2 Antibody Pair [HRP]
This product is for research use only and is not approved for use in humans or in clinical diagnosis. Antibody Pairs are guaranteed for 6 months from date of receipt.
Loading...
Loading...
Loading...
Loading...