Human CXCL7/NAP-2 ELISA Kit (Colorimetric)
Novus Biologicals, part of Bio-Techne | Catalog # NBP2-59965

Key Product Details
Sample Type & Volume Required Per Well
Serum, plasma and tissue homogenates (50 - 100 uL)
Sensitivity
19.5 pg/mL
Assay Range
78 pg/mL - 5000 pg/mL
Product Specifications
Assay Type
Sandwich ELISA
Kit Type
ELISA Kit (Colorimetric)
Reactivity
Human
Specificity
This assay has high sensitivity and excellent specificity for detection of human NAP-2. No significant cross-reactivity or interference between human NAP-2 and analogues was observed.
Description
Assay Length: 1-5 hours
Precision
Intra-Assay Precision (Precision within an assay) %CV < 8
Inter-Assay Precision (Precision between assays) %CV < 10
Scientific Data Images for Human CXCL7/NAP-2 ELISA Kit (Colorimetric)
ELISA: Human CXCL7/NAP-2 ELISA Kit (Colorimetric) [NBP2-59965]
Human CXCL7/NAP-2 ELISA Kit (Colorimetric) [NBP2-59965] -
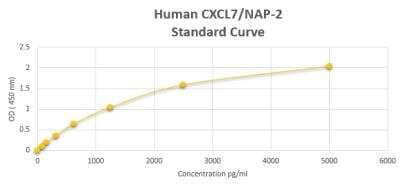
Human CXCL7/NAP-2 ELISA Kit (Colorimetric) [NBP2-59965] - Standard Curve ReferenceKit Contents for Human CXCL7/NAP-2 ELISA Kit (Colorimetric)
- Adhesive Strip (For 96 wells)
- Assay plate (12 x 8 coated Microwells)
- Biotin-antibody (100 x concentrate)
- Biotin-antibody Diluent
- HRP-avidin (100 x concentrate)
- HRP-avidin Diluent
- Instruction manual
- Sample Diluent
- Standard (Freeze dried)
- Stop Solution
- TMB Substrate
- Wash Buffer (25 x concentrate)
Preparation and Storage
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below.
Stability & Storage
Storage of components varies. See protocol for specific instructions.
Background: CXCL7/NAP-2
Alternate Names
CTAP III, NAP-2, NAP2, PPBP
Gene Symbol
PPBP
Additional CXCL7/NAP-2 Products
Product Documents for Human CXCL7/NAP-2 ELISA Kit (Colorimetric)
Product Specific Notices for Human CXCL7/NAP-2 ELISA Kit (Colorimetric)
This product is for research use only and is not approved for use in humans or in clinical diagnosis. ELISA Kits are guaranteed for 6 months from date of receipt.
Loading...
Loading...
Loading...
Loading...
![Human CXCL7/NAP-2 ELISA Kit (Colorimetric) [NBP2-59965] - Human CXCL7/NAP-2 ELISA Kit (Colorimetric)](https://resources.bio-techne.com/images/products/nbp2-59965_human-cxcl7-nap-2-elisa-kit-colorimetric-3120248485440.png)