RABEP2 293T Cell Transient Overexpression Lysate, Novus Biologicals
Bio-Techne includes Novus Biologicals | Catalog # H00079874-T01
Key Product Details
Species
Human
Applications
Western Blot
Product Summary for RABEP2 293T Cell Transient Overexpression Lysate
Quality control test: Transient overexpression cell lysate was tested with Anti-RABEP2 antibody by Western Blots. Plasmid: pCMV-RABEP2 full-length
Product Specifications
Specificity
RABEP2 293T Cell Transient Overexpression Lysate(Denatured)
Type
293T Cell Transient Overexpression
Protein State
Denatured
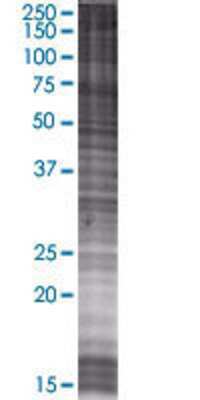
Scientific Data Images for RABEP2 293T Cell Transient Overexpression Lysate
RABEP2 transfected lysate.
Lane 1: RABEP2 transfected lysate (58.74 KDa) Lane 2: Non-transfected lysate.
Formulation, Preparation, and Storage
Formulation
1X Sample Buffer (50 mM Tris-HCl, 2% SDS, 10% glycerol, 300 mM 2-mercaptoethanol, 0.01% Bromophenol blue)
Concentration
mg/ml
Shipping
The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below.
Storage
Store at -80C. Avoid freeze-thaw cycles.
Background: RABEP2
Alternate Names
FLJ23282, FRA, rab GTPase-binding effector protein 2, rabaptin, RAB GTPase binding effector protein 2, Rabaptin-5 beta, Rabaptin-5beta, RABPT5B
Gene Symbol
RABEP2
Additional RABEP2 Products
Product Documents for RABEP2 293T Cell Transient Overexpression Lysate
Product Specific Notices for RABEP2 293T Cell Transient Overexpression Lysate
This product is produced by and distributed for Abnova, a company based in Taiwan.
This product is for research use only and is not approved for use in humans or in clinical diagnosis. Lysates are guaranteed for 6 months from date of receipt.
Loading...
Loading...
Loading...
Loading...