TRAILR3/TNFRSF10C 293T Cell Transient Overexpression Lysate, Novus Biologicals
Bio-Techne includes Novus Biologicals | Catalog # H00008794-T01
Key Product Details
Species
Human
Applications
Western Blot
Product Summary for TRAILR3/TNFRSF10C 293T Cell Transient Overexpression Lysate
Quality control test: Transient overexpression cell lysate was tested with Anti-TNFRSF10C antibody by Western Blots. Plasmid: pCMV-TNFRSF10C full-length
Product Specifications
Specificity
TRAILR3/TNFRSF10C 293T Cell Transient Overexpression Lysate(Denatured)
Type
293T Cell Transient Overexpression
Protein State
Denatured
Scientific Data Images for TRAILR3/TNFRSF10C 293T Cell Transient Overexpression Lysate
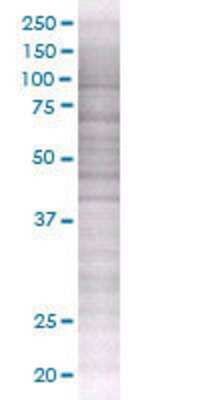
TNFRSF10C transfected lysate.
Lane 1: TNFRSF10C transfected lysate (27.4 KDa) Lane 2: Non-transfected lysate.
Formulation, Preparation, and Storage
Formulation
1X Sample Buffer (50 mM Tris-HCl, 2% SDS, 10% glycerol, 300 mM 2-mercaptoethanol, 0.01% Bromophenol blue)
Concentration
mg/ml
Shipping
The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below.
Storage
Store at -80C. Avoid freeze-thaw cycles.
Background: TRAIL R3/TNFRSF10C
Long Name
TRAIL Receptor 3
Alternate Names
CD263, DcR1, TNFRSF10C, TRAILR3
Gene Symbol
TNFRSF10C
Additional TRAIL R3/TNFRSF10C Products
Product Documents for TRAILR3/TNFRSF10C 293T Cell Transient Overexpression Lysate
Product Specific Notices for TRAILR3/TNFRSF10C 293T Cell Transient Overexpression Lysate
This product is produced by and distributed for Abnova, a company based in Taiwan.
This product is for research use only and is not approved for use in humans or in clinical diagnosis. Lysates are guaranteed for 6 months from date of receipt.
Loading...
Loading...
Loading...
Loading...