Recombinant Human Asparagine Synthetase/ASNS His Protein, CF
R&D Systems, part of Bio-Techne | Catalog # 10193-AS

Key Product Details
Product Specifications
Source
Human embryonic kidney cell, HEK293-derived human Asparagine Synthetase/ASNS protein
Cys2-Ala561
with C-terminal 6-His tag
Cys2-Ala561
with C-terminal 6-His tag
Purity
>95%, by SDS-PAGE visualized with Silver Staining and quantitative densitometry by Coomassie® Blue Staining.
Endotoxin Level
<1.0 EU per 1 μg of the protein by the LAL method.
N-terminal Sequence Analysis
Cys
Predicted Molecular Mass
65 kDa
SDS-PAGE
58-65 kDa, under reducing conditions
Activity
Measured by its ability to produce diphosphate during the conversion of aspartate and glutamine to asparagine and glutamate.
The specific activity is >100 pmol/min/μg, as measured under the described conditions.
The specific activity is >100 pmol/min/μg, as measured under the described conditions.
Scientific Data Images for Recombinant Human Asparagine Synthetase/ASNS His Protein, CF
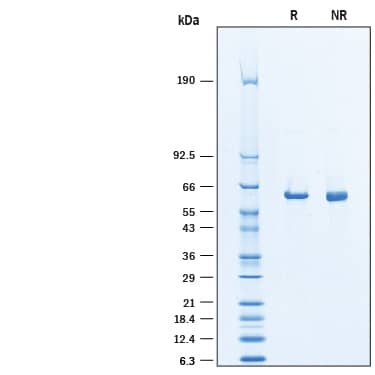
Recombinant Human Asparagine Synthetase/ASNS His Protein SDS-PAGE
2 μg/lane of Recombinant Human Asparagine Synthetase/ASNS His-tag was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® blue staining, showing a band at ~62 kDa under reducing conditions.Formulation, Preparation and Storage
10193-AS
| Formulation | Supplied as a 0.2 μm filtered solution in Tris, NaCl, TCEP and Glycerol. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: Asparagine Synthetase/ASNS
References
- Richards, N. G. J. and Schuster, S.M. (1998) Adv. Enzymol. 72:145.
- Zalkin, H. and J. L. Smith (1998) Adv. Enzymol. Relat. Areas Mol. Biol. 72:87.
- Larsen, T. M. et al. (1999) Biochemistry. 38:16146
- Milman, H. A. and D. A. Cooney (1974) Biochem. J. 142:27.
- Lomelino, C. L. et al. (2017) J. Biol. Chem. 292:19952.
- Ruzzo, E. K. et al. (2013) Neuron. 80:429.
- Hawkins, R. A. et al. (2006) J. Nutr. 136:218S.
- Haskell, C. M. et al. (1969) Biochem. Pharmacol. 18:2578.
- Aslanian, A. M. et al. (2001) Biochem. J. 357:321.
- Su, N. et al. (2008) Pediatr. Blood Cancer. 50:274.
- Ye, J. et al. (2010) EMBO J. 29:2082.
- Pieters, R. et al. (2011) Cancer 117:238.
- Lorenzi, P. L. et al. (2008) Mol. Cancer Ther. 7:3123.
- Dufour, E. et al. (2012) Pancreas. 41:940.
- Du, F. et al. (2019) Cell Death Dis. 10:239.
Long Name
ASNS
Alternate Names
ASNSD, EC 6.3.5.4, Glutamine Dependent Asparagine Synthetase, TS11 Cell Cycle Control Protein
Gene Symbol
ASNS
UniProt
Additional Asparagine Synthetase/ASNS Products
Product Documents for Recombinant Human Asparagine Synthetase/ASNS His Protein, CF
Product Specific Notices for Recombinant Human Asparagine Synthetase/ASNS His Protein, CF
For research use only
Loading...
Loading...
Loading...