Recombinant Human/Bovine Gremlin 1, Animal-Free Protein
R&D Systems, part of Bio-Techne | Catalog # Qk015

Key Product Details
Product Specifications
Source
Purity
Endotoxin Level
Predicted Molecular Mass
SDS-PAGE
Activity
Mycoplasma
Scientific Data Images for Recombinant Human/Bovine Gremlin 1, Animal-Free Protein
Recombinant Human/Bovine Gremlin 1, Animal-Free Protein Bioactivity
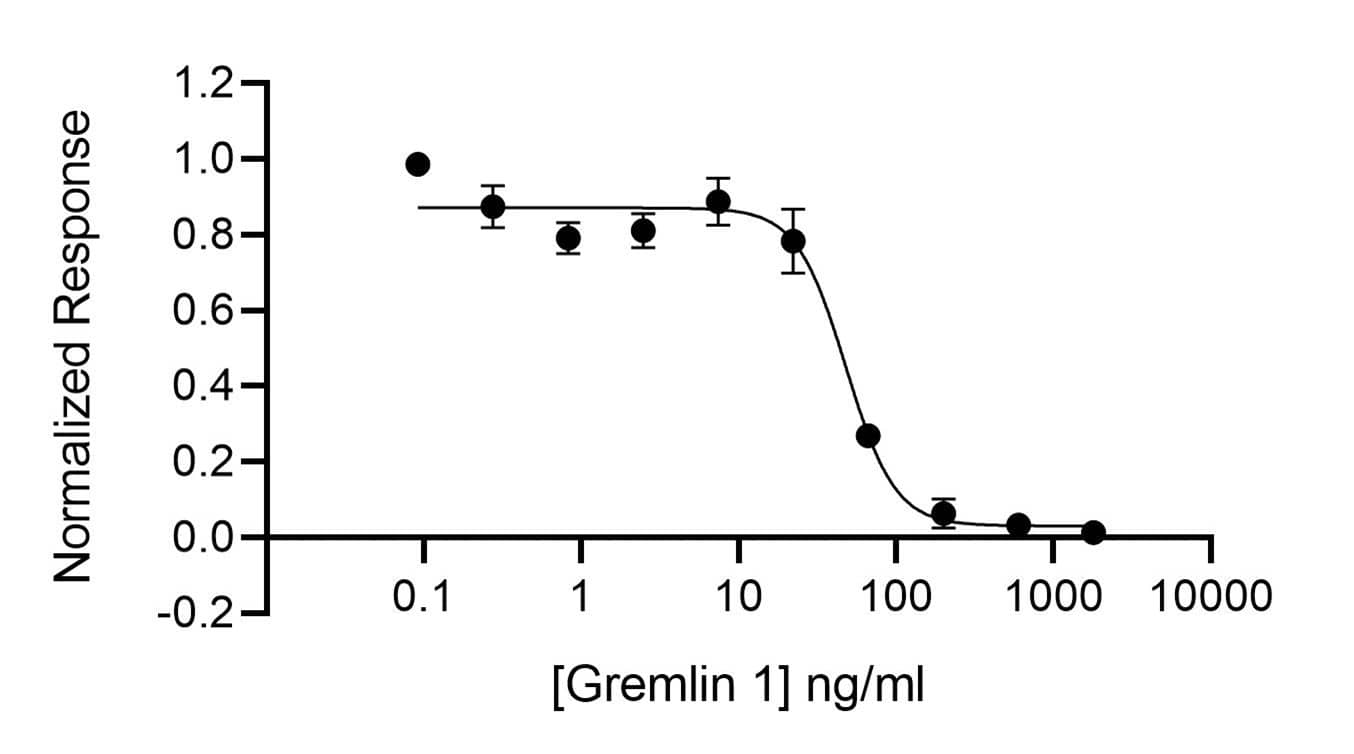
Gremlin 1 activity is determined using inhibition of the BMP2 response (Qk007 #010, 52 ng/ml) from a BMP2-responsive firefly luciferase reporter in stably transfected HEK293T cells. Cells are treated (n=4) with a serial dilution of Gremlin 1 in BMP2 for 6 hours. Firefly luciferase activity is measured and normalized to the control Renilla luciferase activity.Gremlin-1 inhibits BMP-2 induced luciferase activity with an EC50 = 47.9 ng/ml (2.66 nM).Recombinant Human/Bovine Gremlin 1, Animal-Free Protein SDS-PAGE
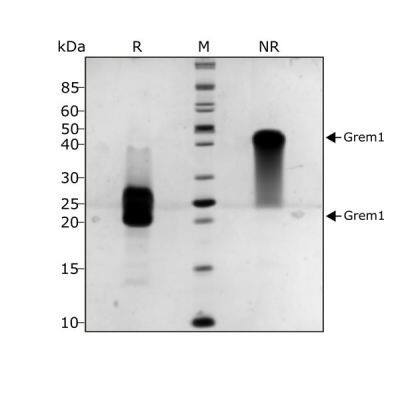
Gremlin 1 protein migrates as a single diffuse band at ~36 kDa in non-reducing (NR) and 19 kDa in reducing (R) conditions. The protein is a non-covalent dimer and it is the dissociation of the dimer during electrophoresis which gives the characteristic diffuse band.Purified recombinant protein (7 µg) was resolved using 15% w/v SDS-PAGE in reduced (+ beta-mercaptoethanol, R) and non-reduced conditions (NR) and stained with Coomassie Brilliant Blue R250.Formulation, Preparation and Storage
Qk015
| Formulation | Lyophilized from acetonitrile/TFA |
| Reconstitution | Resuspend in 10 mM HCl at >100 µg/ml, prepare single use aliquots, add carrier protein if desired. |
| Shipping | The product is shipped lyophilized at ambient temperature, on ice blocks or on dryice. Shipping at ambient temperature does not affect the bioactivity or stability ofthe protein. Upon receipt, store immediately at the conditions stated below. |
| Stability & Storage | Store lyophilized protein between -20 °C and -80 °C until the date of expiry.Avoid freeze-thaw cycles. |
Background: Gremlin
Gremlin, also known as Increased in High Glucose protein 2 (IHG-2) and Down-regulated in Mos-transformed cells protein (Drm), is a 28 kDa member of the Dan family of secreted glycoproteins (1-3). Human Gremlin is synthesized as a 184 amino acid (aa) precursor that contains a 24 aa signal sequence and a 160 aa mature region (SwissProt # O60565). The mature region contains one potential site for N-linked glycosylation (Asn 42), a cysteine-rich region, and a cysteine-knot motif (aa 94‑184) whose structure is shared by members of the TGF-beta superfamily (3). Post-translational modifications include glycosylation and phosphorylation (3). Gremlin exists in both secreted and membrane-associated forms (3). There are two isoforms for human Gremlin. Isoform 1 is the standard protein, and in isoform 2, there is a deletion of aa 39‑79. Human Gremlin shares 99% and 86% aa sequence identity with mouse and chick Gremlin, respectively. Northern blot analysis shows that Gremlin mRNA is highly expressed in the small intestine, fetal brain and colon, and weakly expressed in adult brain, ovary, prostate, pancreas and skeletal muscle (4). Gremlin functions as a bone morphogenetic protein (BMP) antagonist. It acts by binding to, and forming heterodimers with, BMP-2, BMP-4, and BMP-7, thus preventing them from interacting with their cell surface receptors (1). This mechanism is thought to be responsible for the pattern-inducing activity of Gremlin during embryonic development (5) and to play a role in human diseases, such as diabetic nephropathy (6). However, intracellular BMP-independent mechanisms of action (7) may mediate the ability of Gremlin to suppress transformation and tumorigenesis under certain experimental conditions (8-9). Gremlin also interacts with Slit proteins and acts as an inhibitor of monocyte chemotaxis (10). In addition, Gremlin has been found to be a proangiogenic factor expressed by endothelium (9).
References
- Hsu, D.R. et al. (1998) Mol. Cell 1:673.
- McMahon, R. et al. (2000) J. Biol. Chem. 275:9901.
- Wordinger, R.J. et al. (2008) Exp. Eye Res. 87:78.
- Topol, L.Z. et al. (2000) Cytogenet. Cell Genet. 89:79.
- Khokha, M.K. et al. (2003) Nat. Genet. 34:303.
- Lappin, D.W. et al. (2002) Nephrol. Dial. Transplant 17:65.
- Chen, B. et al. (2002) Biochem. Biophys. Res. Commun. 295:1135.
- Topol, L.Z. et al. (1997) Mol. Cell. Biol. 17:4801.
- Stabile, H. et al. (2007) Blood 109:1834.
- Chen, B. et al. (2004) J. Immunol. 173:5914.
Alternate Names
Gene Symbol
UniProt
Additional Gremlin Products
Product Documents for Recombinant Human/Bovine Gremlin 1, Animal-Free Protein
Product Specific Notices for Recombinant Human/Bovine Gremlin 1, Animal-Free Protein
The above product was manufactured, tested and released by R&D System's contract manufacturer, Qkine Ltd, at 1 Murdoch House, Cambridge, UK, CB5 8HW. The product is for research use only and not for the diagnostic or theraputic use.
For research use only