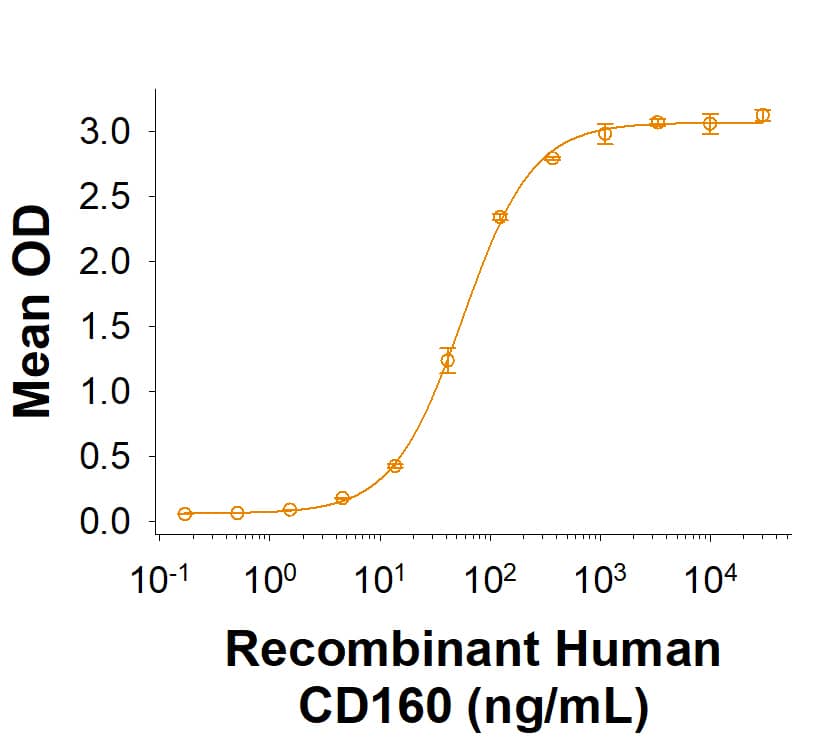
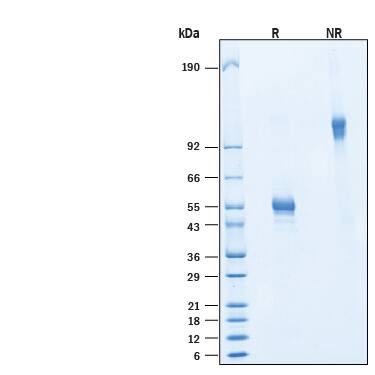
CD160 (also Natural killer cell receptor BY55) is a 27 ‑ 30 kDa member of the Ig superfamily (1 ‑ 4). In human, it is expressed principally on nonmyeloid hematopoietic cells. These include CD56DIMCD16+ cytolytic NK cells, CD8+CD28- T cells, CD8+CD101+ IELs, NKT cells, gamma δ TCR T cells, activated CD4+ T cells, and vascular endothelial cells (1, 5 ‑ 7). CD160 was initially identified as a GPI‑linked glycoprotein (3). It is synthesized as a preproprotein that is 181 amino acids (aa) in length. The precursor contains a 26 aa signal sequence, a 133 aa mature molecule that shows one 96 aa V‑type Ig‑like domain (aa 27 ‑ 122), and a 22 aa prosegment that is cleaved to generate a GPI‑linkage at Ser159. GPI‑linked CD160 is known to be cleaved by phospholipases and these generate an 80 kDa (presumably trimeric) band in SDS‑PAGE (1, 8). Alternative splice forms for CD160 are reported to exist on activated NK cells. The principal variant is an extended type I transmembrane (TM) protein that shows a 55 aa substitution for the C‑terminal two amino acids. It contains a 23 aa TM segment (aa 160 ‑ 182) and a 52 aa cytoplasmic region. Two other variants show deletions of the Ig‑like domain in both the GPI‑linked and TM form (9). Mature human CD160 shares 62% aa identity with mouse CD160.
CD160 is known to bind to HLA-G1, HLA-C, and HVEM (6, 9, 10). And upon engagement, it is reported to associate with CD2 in cis under certain conditions (11, 12). The effects of CD160 ligation appear to be context dependent. When expressed on endothelial cells, CD160 binding to HLA-G1 initiates apoptosis, and thus impacts angiogenesis (6). When expressed on CD56DIM NK cells, CD160 signaling in response to HLA-C binding promotes IFN-gamma, TNF-alpha, and IL-6 secretion (10). And when up‑regulated on CD4+ T cells following activation, CD160 engagement by HVEM (expressed by APC) serves to block a simultaneous LIGHT stimulation of HVEM that promotes receptor expression and cytokine release (1, 2, 7, 13).