Recombinant SARS-CoV-2 Spike Avi-tag His-tag Protein CF
R&D Systems, part of Bio-Techne | Catalog # AVI10586

Key Product Details
Source
Accession #
Structure / Form
Conjugate
Applications
Product Specifications
Source
| SARS-CoV-2 Spike (Val16-Lys1211)(Arg682Ser, Arg685Ser, Lys986Pro, Val987Pro) Accession # YP_009724390.1 |
Avi-tag | HHHHHH |
| N-terminus | C-terminus |
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
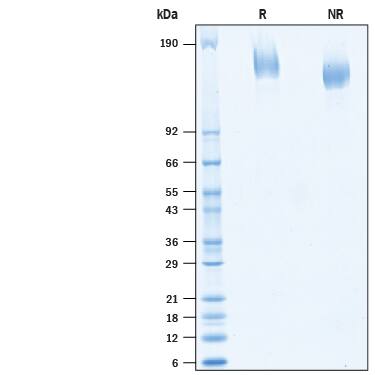
SDS-PAGE
Activity
The biotin to protein ratio is greater than 0.7 as determined by the HABA assay.
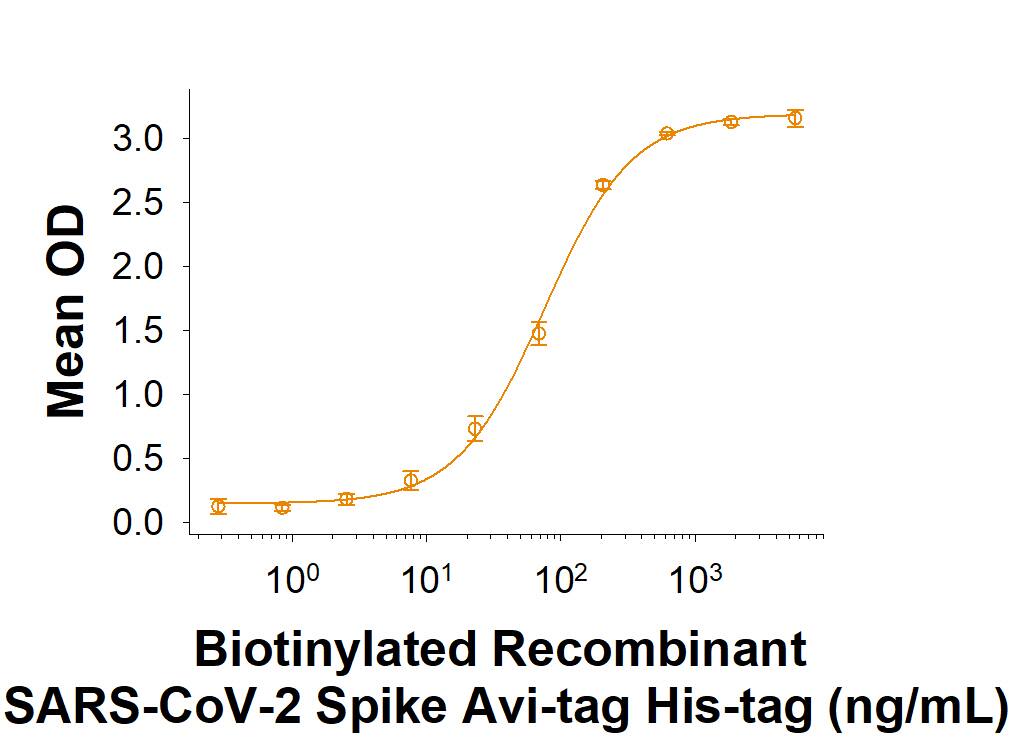
Measured by its binding ability in a functional ELISA with Recombinant Human ACE-2 Fc Chimera (Catalog # 10544-ZN).
Scientific Data Images for Recombinant SARS-CoV-2 Spike Avi-tag His-tag Protein CF
Biotinylated Recombinant SARS-CoV-2 Spike Avi-tag His-tag Protein Binding Activity.
Biotinylated Recombinant SARS-CoV-2 Spike Avi-tag His-tag (Catalog # AVI10586) binds Recombinant Human ACE-2 Fc Chimera (10544-ZN) in a functional ELISA.Biotinylated Recombinant SARS-CoV-2 Spike Avi-tag His Protein SDS PAGE.
2 μg/lane of Biotinylated Recombinant SARS-CoV-2 Spike Avi-tag His Protein (Catalog # AVI10586) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 155-175 kDa.Formulation, Preparation and Storage
AVI10586
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: Spike
SARS-CoV-2, which causes the global pandemic coronavirus disease 2019 (Covid-19), belongs to a family of viruses known as coronaviruses that are commonly comprised of four structural proteins: Spike protein (S), Envelope protein (E), Membrane protein (M), and Nucleocapsid protein (N) (1). SARS-CoV-2 Spike Protein (S Protein) is a glycoprotein that mediates membrane fusion and viral entry. The S protein is homotrimeric, with each ~180-kDa monomer consisting of two subunits, S1 and S2 (2). In SARS-CoV-2, as with most coronaviruses, proteolytic cleavage of the S protein into the S1 and S2 subunits is required for activation. The S1 subunit is focused on attachment of the protein to the host receptor while the S2 subunit is involved with cell fusion (3-5). The S protein of SARS-CoV-2 shares 75% and 29% amino acid (aa) sequence identity with the S protein of SARS-CoV-1 and MERS, respectively.The S Protein of the SARS-CoV-2 virus, like the SARS-CoV-1 counterpart, binds Angiotensin-Converting Enzyme 2 (ACE2), but with much higher affinity and faster binding kinetics through the receptor binding domain (RBD) located in the C-terminal region of S1 (6). Based on structural biology studies, the RBD can be oriented either in the up/standing or down/lying state with the up/standing state associated with higher pathogenicity (7). Polyclonal antibodies to the RBD of the SARS-CoV-2 protein have been shown to inhibit interaction with the ACE2 receptor, confirming RBD as an attractive target for vaccinations or antiviral therapy (8). It has been demonstrated that the S Protein can invade host cells through the CD147/EMMPRIN receptor and mediate membrane fusion (9, 10). A SARS-CoV-2 variant carrying the S protein aa change D614G has become the most prevalent form in the global pandemic and has been associated with greater infectivity and higher viral load (11, 12). Our Avi-tag Biotinylated SARS-CoV-2 Spike protein features biotinylation at a single site contained within the Avi-tag, a unique 15 amino acid peptide. Protein orientation will be uniform when bound to streptavidin-coated surface due to the precise control of biotinylation and the rest of the protein is uncharged so there is no interference in the protein's bioactivity.
References
- Wu, F. et al. (2020) Nature 579:265.
- Tortorici, M.A. and D. Veesler (2019). Adv. Virus Res. 105:93.
- Bosch, B.J. et al. (2003). J. Virol. 77:8801.
- Belouzard, S. et al. (2009) Proc. Natl. Acad. Sci. 106:5871.
- Millet, J.K. and G.R. Whittaker (2015) Virus Res. 202:120.
- Ortega, J.T. et al. (2020) EXCLI J. 19:410.
- Yuan, Y. et al. (2017) Nat. Commun. 8:15092.
- Tai, W. et al. (2020) Cell. Mol. Immunol. https://doi.org/10.1016/j.it.2020.03.007.
- Wang, X. et al. (2020) https://doi.org/10.1038/s41423-020-0424-9.
- Wang, K. et al. (2020) bioRxiv https://www.biorxiv.org/content/10.1101/2020.03.14.988345v1.
- Korber, B. et al. (2020) Cell 182, 812.
- Zhang, L. et al. (2020) bioRxiv https://www.biorxiv.org/content/10.1101/2020.06.12.148726v1.
Long Name
Alternate Names
Entrez Gene IDs
Gene Symbol
UniProt
Additional Spike Products
Product Documents for Recombinant SARS-CoV-2 Spike Avi-tag His-tag Protein CF
Product Specific Notices for Recombinant SARS-CoV-2 Spike Avi-tag His-tag Protein CF
For research use only