
By Jamshed Arslan, Pharm. D., PhD.
What is metabolic syndrome?
It was 1988 when, after decades of research, Dr. GM Reaven of the Stanford University, CA, explained the relationship between four diseases: insulin resistance, hypertension, diabetes and obesity. Since that time, the research on this "deadly quartet" has never stopped. Although Reaven coined the term 'Syndrome X', through the years, others have used terms like Reaven’s syndrome, cardiometabolic syndrome, insulin resistance syndrome, and most commonly, the metabolic syndrome. This syndrome describes a cluster of cardiovascular risk factors: altered glucose metabolism (insulin resistance), hypertension, central obesity and dysregulated lipid metabolism (dyslipidemia). The clinical diagnosis of metabolic syndrome depends on the presence of at least three of the following five conditions: elevated waist circumference, triglycerides, blood pressure and fasting plasma glucose levels, and reduced HDL-cholesterol.
Role of insulin resistance
Dr. Reaven emphasized the role of insulin resistance in developing 'syndrome X'. Today, insulin resistance and associated dysfunctional lipolysis are at the center of the metabolic syndrome pathophysiology. Insulin resistance is considered a common feature of atherosclerosis, nonalcoholic fatty liver disease and most importantly, Type 2 diabetes.
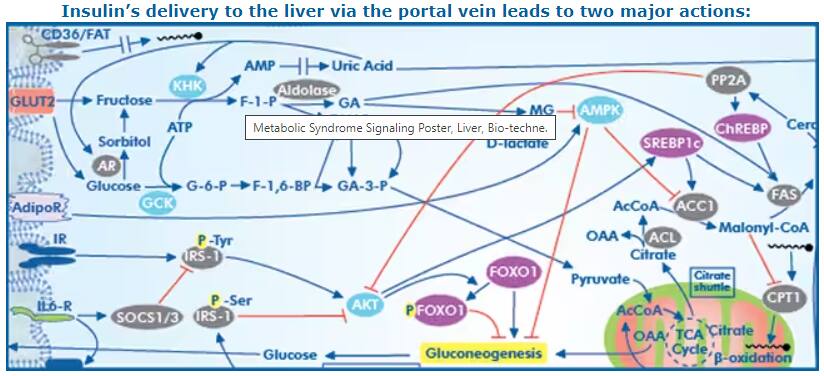
- Decreased glucose output: Insulin inhibits the expression of gluconeogenesis-associated genes through phosphorylation of the transcription factor FoxO1.
- Increased de novo lipogenesis (DNL): Insulin activates transcription factor SREBP-1c, which leads to increased expression of genes associated with fatty acid and triglycerides biosynthesis.
Diagram from Metabolic Syndrome Signaling Poster, published in its original form in a collaborative between Dr. Robert H. Lustig, Dr. Alejandro Gugliucci and Bio-Techne Corporation
As the response to normal insulin levels decreases in the main target organs (liver, skeletal muscles and adipocytes), beta cells of pancreatic islet of Langerhans secrete more insulin to compensate. Hyperglycemia with hyperinsulinemia increases overall hepatic glycogen synthesis. Later, decompensation occurs, which reduces hepatic glycogenolysis. This effect is underscored by increased blood glucose, which without correspondingly high insulin, inhibits glycogen phosphorylase. It remains unclear why insulin resistance impairs glucose homeostasis mechanisms while concomitantly increasing hepatic de novo lipogenesis. However, it's clear that free fatty acids (FFAs) stimulate production of triglycerides, glucose and atherogenic very low-density LDL.
Increased plasma FFAs due to liver insulin resistance, disrupts glucose-fatty acid (Randle) cycle and insulin-dependent glucose uptake by muscles. This facilitates hyperglycemia and ectopic deposition of fats in muscles (intramyocellular lipid), thereby contributing to the metabolic syndrome.
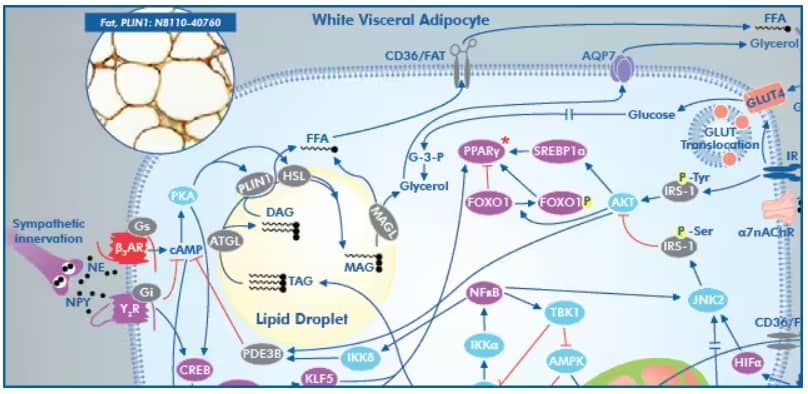
Visceral adipose tissue is responsive to adrenergic innervation, activation of B3AR leads to concomitant activation of hormone-sensitive lipase (HSL) to promote lipolysis.
Diagram from Metabolic Syndrome Signaling Poster, published in its original form in a collaborative between Dr. Robert H. Lustig, Dr. Alejandro Gugliucci and Bio-Techne Corporation
Insulin prevents adipose tissue lipolysis. Therefore, decreased adipose tissue response to insulin leads to increased plasma FFAs, particularly from the catecholamine-mediated lipolysis in visceral adipocytes. It should be noted that subcutaneous fat is also strongly associated with insulin resistance in diabetic as well as healthy obese individuals.
Contribution of obesity in metabolic syndrome
One paradigm that links obesity to metabolic syndrome suggests that obesity disrupts the normal lipid storage patterns, leading to elevated FFAs and inflammatory cytokines (TNF-alpha and IL-6), and reduced adiponectin, a hormone produced by adipocytes that regulates glucose levels and fatty acid breakdown. The net effect is insulin resistance (altered glucose metabolism), atherogenesis (endothelial dysfunction) and cardiovascular disease.
Markers of metabolic syndrome
Insulin resistance and central obesity are considered the major players in the development of cardiovascular risk factors known as metabolic syndrome. However, the fact that many lean and non-diabetic individuals also suffer from metabolic syndrome indicate a key fact: obesity and diabetes are markers, not the cause of the metabolic syndrome.

Jamshed Arslan, Pharm D, PhD
Dr. Arslan is an Assistant Professor at Dow University of Health Sciences, Pakistan,
where he teaches Pharmacology to future pharmacists.
-
Punthakee, Z. et al. (2018) Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Canadian Journal of Diabetes.
-
McCracken, E. et al. (2018) Pathophysiology of the metabolic syndrome. Clinics in Dermatology
-
Reaven, G.M. (1988) Role of insulin resistance in human disease. Diabetes.
-
Weiss, R. et al. (2013) What is metabolic syndrome, and why are children getting it? Annals of the New York Academy of Sciences.