Analysis of Insulin by Maurice
Summary
The insulin pro-peptide consists of a 21-amino-acid A chain, a 30-amino-acid B chain, and a C peptide. Removal of the C peptide by proteolysis enables the formation of mature Insulin, a disulfide-linked heterodimer of the A and B chains. Commercially produced insulin analogs are frequently used to treat diabetes and exist in several forms that alter the onset of action. Insulin lispro is a fast-acting insulin analog that has the terminal lysine and proline residues reversed. Insulin glargine is longer acting due to its slower release.
USP catalogue numbers
Insulin lispro: 1342321, Lot R016U0
Insulin glargine: 1342059, Lot F009M0
Human insulin: 1342106, Lot R032L0
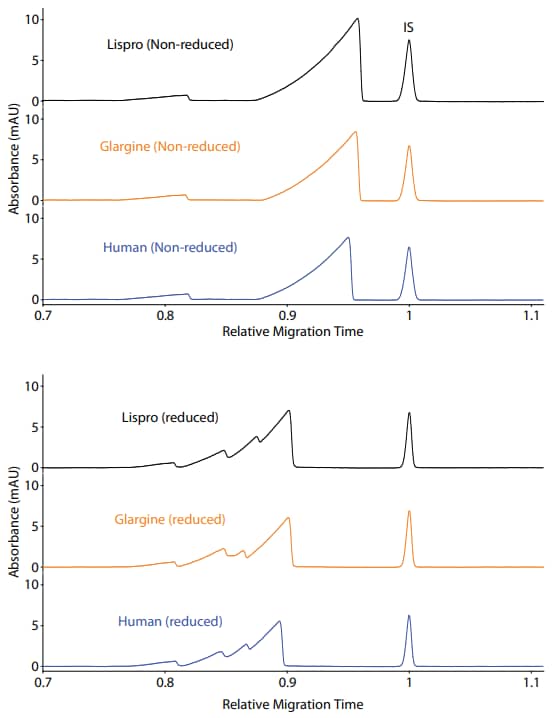
Maurice CE-SDS Method
Sample preparation: Human insulin, insulin lispro, and insulin glargine were diluted to 0.5 mg/mL in 1X Maurice Sample Buffer prior to treatment for 5 minutes at 70 °C in the presence of either 11.5 mM IAM (non-reduced) or 650 mM bME (reduced).
Running conditions: Non-reduced insulin samples were injected for 20 seconds followed by a 15-minute separation at 4600 V. Reduced insulin samples were injected for 10 seconds followed by a 15-minute separation at 4600 V.
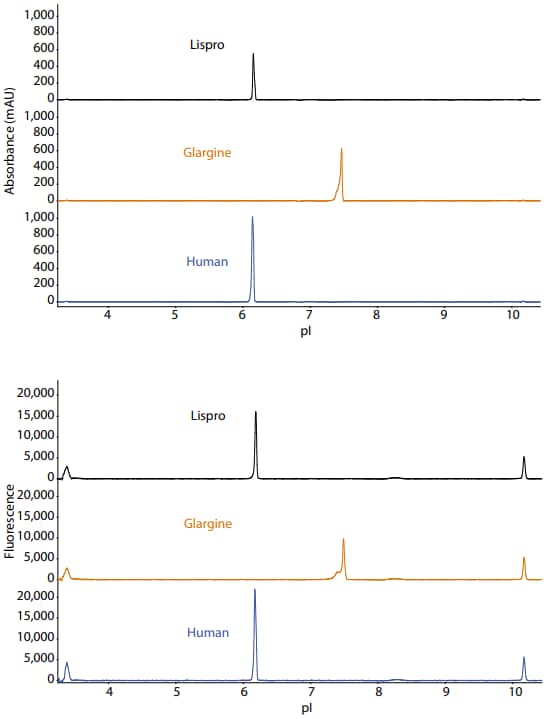
Maurice icIEF method
Sample preparation: Insulins were diluted to 0.05 mg/mL.
Ampholyte solution: Pharmalytes 3–10 (4%) containing 12.5 mM arginine, 12.5 mM IDA and 3.2 M urea
Running conditions: 1 minute at 1500 V, 6 minutes at 3000 V
pI markers: 3.38 and 10.17
Imaging: Absorbance and fluorescence (30 seconds)