Bio-Techne: Your Partner from Protein Biomarker Discovery to Commercialization
Bio-Techne offers a robust suite of fit-for-purpose assay development and validation services, utilizing a broad portfolio of in-house proteins, antibodies, and immunoassays of industry-leading quality, eliminating commercial barriers and uncertainty in long-term supply and continuity of services.
With multiple service labs operating under GxP standards for RUO to IUO through IVD assay development, CLIA labs for clinical trial sample testing, a dedicated regulatory team to support concurrent drug/CDx filing, and a global footprint, Bio-Techne offers a true end-to-end partnership for your translational biomarker journey.
- Early strategic alignment with stakeholders to efficiently execute a program
- Phased approach with defined stage-gate deliverables to allow for flexibility
- Timeline alignment to ensure co-development of IVD and therapeutic for contemporaneous approval
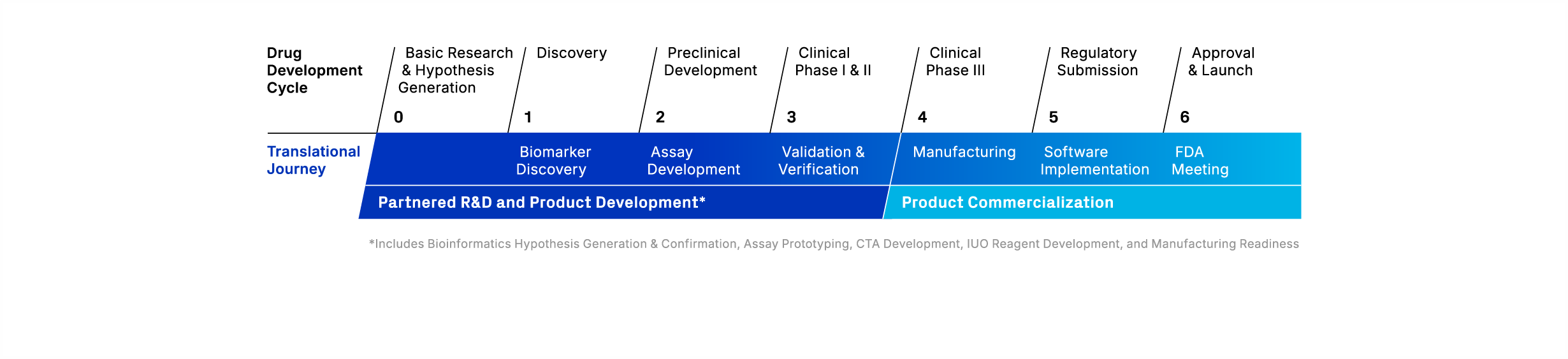
Biomarker Development Workflow
We offer precision medicine services spanning the continuum from biomarker discovery to commercialization of companion diagnostic products. Our customizable solutions improve your drug’s likelihood to succeed and deliver novel treatments to patients most likely to benefit, faster.
- Flexibility to build strategies with innovative technologies or established platforms
- Broad portfolio of best-in-class products for custom fit-for-purpose clinical trial assay development
- Clinical trial sample testing readiness at our CLIA labs
- Decentralized solutions with kitted products (cGMP)
- International regulatory expertise for designated risk classifications in geographies of interest
- Experience on reimbursement strategies to lower patient access barriers
Proteomic Biomarker Signature Discovery
Olink Gold-certified service provider for high throughput Proximity Extension Assay (PEA) targeted protein biomarker analysis, enabling the discovery of multi-protein signatures.
Integrated Analytics utilizing our state-of-the-art bioinformatics and machine learning algorithms to provide a multifaceted view of human health and disease.
Luminex® multiplex immunoassays validate multiple biomarker candidates by profiling up to 50 proteins in one sample.
Targeted Protein Biomarker Assay Development and Validation
R&D Systems™ cGMP antibodies, proteins, and specialized diluents, the foundation of our immunoassays with quality systems in place to bring into the clinic as a fit-for-purpose assay to support clinical trials.
Quantikine™ and Duoset™ ELISAs for superior performance and reproducibility; the most referenced ELISAs in literature.
Ella ™ Simple-Plex™ next-generation benchtop automated ELISA platform designed to deliver accurate, reproducible data with no manual steps.
Leo and Jess™ Simple Western™ capillary-based immunoassay systems for fully automated Western blot analysis.
Clinical Testing and Regulatory Support
CAP-accredited CLIA-certified Labs to support clinical testing needs with validated assays (IUO, CTA, LDT, IVD).
Robust Quality Management System certified to ISO 13485:2016, MDSAP and IVDR (EU 2017/746).
Proven Regulatory Expertise with successful IVD registrations across the globe, including Australia, Canada, Europe, Korea, the United Kingdom, and the United States.
Commercialization Success with direct sales and technical support.
Decentralized Solutions provide market access with kitted cGMP manufacturing in our FDA-registered ISO 13485 facilities.
Simplicity of Licensing for technology access, minimizing bridging studies and eliminating commercial barriers.
Global Presence with 34 locations across US, EMEA, and APAC and existing commercial channels in over 50 countries.
Multiomic Approach to Precision Medicine
The proteomic capabilities at Bio-Techne are one component of our diverse precision medicine offering supporting biomarker discoveries in broad modalities through fit-for-purpose clinical assay and CDx development deliverables.
Exosome Technologies and Expertise in proprietary exosome isolation technologies coupled with specialized expertise in developing clinical-grade assays utilizing exosomal analytes extracted from liquid biopsy samples.
Integrated Multiomics Platform that leverages robust bioinformatics and machine learning capabilities for genomic, epigenomic, transcriptomic, and proteomic analysis to support your biomarker strategy.
Ability to Unlock Challenging Regions of DNA and RNA with proprietary chemistries that can analyze a wide range of genomic targets with unprecedented ease. Capabilities extend to complex single nucleotide variants, pseudogene discrimination, methylation, and more.

Frequently Asked Questions
Protein biomarkers provide measurable, real-time indicators of an individual's physiological state, enabling more accurate disease diagnosis, prognosis, and personalized treatment selection based on a patient's unique molecular profile.
Yes. Proteomics enhances personalized medicine by providing crucial insights into individual patient biology, disease mechanisms, and treatment responses, ultimately leading to more precise and effective healthcare.
- Biomarker Discovery: Identifies protein markers for early disease detection and prognosis.
- Pharmacoproteomics: Studies protein-drug interactions, aiding in treatment selection and monitoring.
- Integrated multiomics: Combines with genomics and transcriptomics for comprehensive disease understanding.
- Therapeutic Monitoring: Tracks treatment efficacy through protein expression changes.
- Precision Diagnostics: Enables more accurate disease classification and patient stratification.
The validation of protein biomarkers for clinical testing is a multi-step process that involves both analytical and clinical validation to ensure their reliability and utility in a clinical setting. This process is essential for translating biomarker discoveries into practical tools that can improve patient care.
Steps in the Validation Process
- Defining Biomarkers and Purpose: Initial identification and proof-of-concept studies to define the biomarker and its intended use.
- Assay Development and Optimization: Designing and optimizing the assay, including selecting appropriate reagents and probes, and minimizing cross-reactivity.
- Analytical Validation: Conducting rigorous testing to establish the assay's performance characteristics.
- Clinical Validation: Conducting studies to demonstrate the biomarker's clinical relevance and utility.
- Documentation and Continuous Improvement: Documenting all validation steps and continuously improving the assay based on new data and technological advancements.
Regulatory Considerations
- Submission to Regulatory Agencies: Providing comprehensive data on both analytical and clinical validation to regulatory bodies such as the US FDA.
- Compliance with Standards: Ensuring that the biomarker assay is performed in a CLIA-certified laboratory and consistently meets all regulatory standards for clinical diagnostics
Partnering with Bio-Techne can accelerate assay development, ensure compliance, enhance your market strategy, and ultimately improves patient care in precision medicine.
Benefits of Bio-Techne Partnerships for CDx:
- Specialized Expertise: Access to diagnostic development and validation know-how
- Regulatory Experience: Streamlined navigation of complex approval processes with successful IVD registrations across the globe, including Australia, Canada, Europe, Korea, the United Kingdom, and the United States
- Cost-Effectiveness: Avoid building expensive in-house capabilities
- Technological Access: Leverage our innovative proteomic, multiomic, and exosome isolation and analyte enrichment technologies, our established immunoassay platforms, and best in class antibody and protein reagents
- Market Penetration: Utilize our established global channels and commercialization networks
- Improved Outcomes: Better patient selection for targeted therapies
- Broad Portfolio: Our broad portfolio provides access to proprietary chemistries including liquid biopsy, which offer a unique avenue for protein biomarker discovery
