What is Companion Diagnostics?
A Companion Diagnostic (CDx) assay, according to the The United States Food and Drug Administration guidance, is a medical device, often an in vitro diagnostic (IVD), which provides information that is essential for the safe and effective use of a corresponding drug or biological product. A CDx assay is required when the drug/biologic has a specific genetic or biological target not present in all patients with a particular disease.
Companion diagnostic tests can be used to identify patients most likely to respond to a therapeutic drug or biologic, as well as patients at lower or higher risk for a particular side effect. In addition, a CDx assay can be used to monitor response to treatment with a particular therapeutic product for the purpose of adjusting treatment to achieve improved safety or effectiveness.
Bio-Techne is a trusted partner in companion diagnostics for oncology, neurodegenerative diseases, & beyond.
IVD Product Development
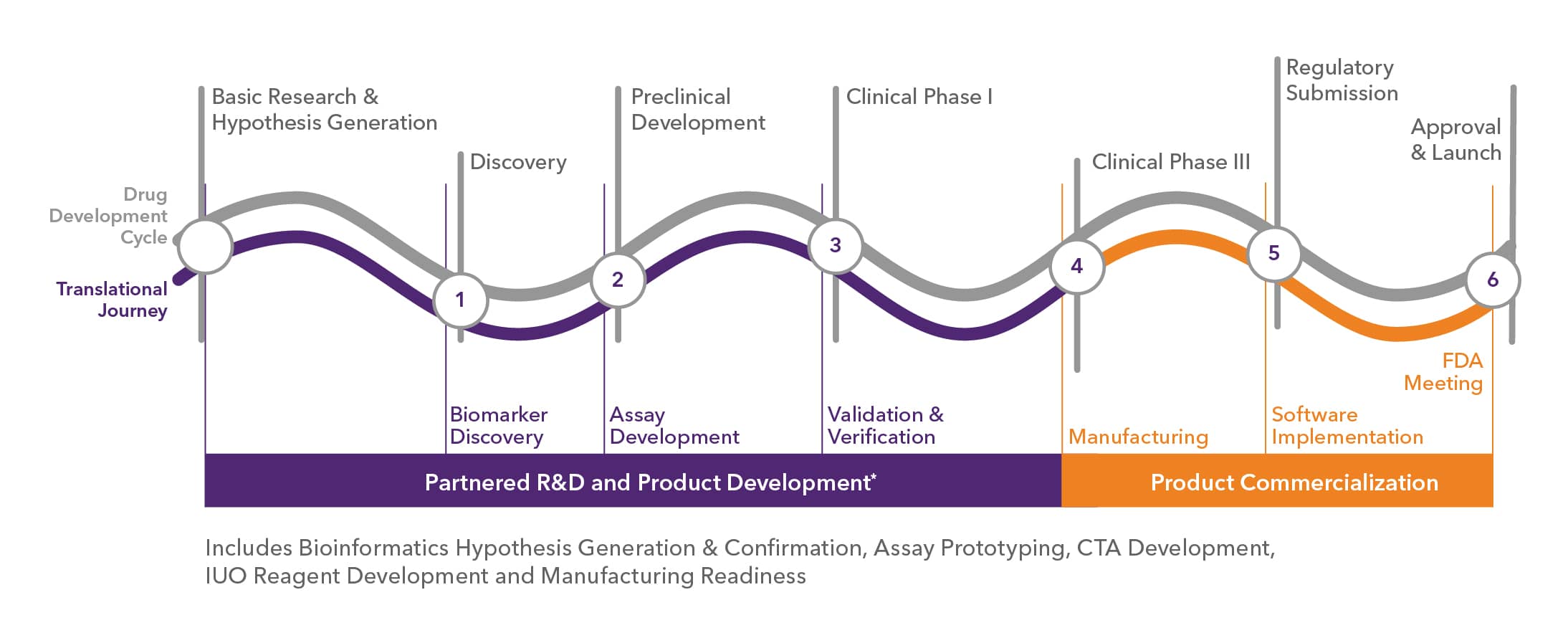
Historically drug manufacturers have faced the challenge of knowing the need for Companion Diagnostics but having to mitigate risks by delaying decisions to engage an IVD partner. This approach was predicated on the knowledge that IVD companies did not generally have the skills needed to support a drug's lifecycle from concept to commercialization. As such, misalignment between drug and CDx development timelines often resulted in enrollment of CDx partners only occurring in Phase II/III causing commercialization delays.
At Bio-Techne, our partnership model is different as we built capabilities and competencies to support partnering with you from concept to commercialization. This approach enables you to engage an IVD partner early in a stage-gated approach that ensures that when you are ready to accelerate, we as a partner already have the foundation that allows us to execute rapidly on a companion Dx and match the pace of your drug.
Benefits of Partnering with Bio-Techne
- Expertise: Bio-Techne brands offer an extensive history in developing and manufacturing high-quality reagents, Dx controls, and immunoassays. In addition, we develop and commercialize diagnostic kits, ranging from the ExoDx Prostate Test for assessing the risk of high-grade prostate cancer to the Asuragen, a Bio-Techne brand's catalog of PCR RUO to CE-IVD assays for a wide range of genetic disorders. Our combined expertise in reagent manufacturing and Dx development enables us to be a trusted partner in fit-for-purpose assay development to accelerate your pharmaceutical clinical trials.
- Quality: R&D Systems™ antibodies, proteins, and specialized diluents are the foundation of our immunoassays. Years of stringent quality control in every raw material and kit culminates in our global industry-leading reputation. In addition, we have the quality systems in place to bring an immunoassay into the clinic as a fit-for-purpose assay to support clinical trials.
- Better Answers, Faster: The use of our in-house reagent and immunoassay portfolios accelerates the timeline from discovery to assay development, enabling the deployment of an analytically validated assay for clinical sample testing at speed.
- Capacity to Scale: Our end-to-end solution utilizing our in-house reagents and immunoassays removes licensing barriers to commercialization. We offer cGMP manufacturing facilities combined with kitted solutions and global sales and support teams to enable the flexibility to deploy our assays in a decentralized setting.