CRISPR-Cas9 Antibody Pack
Novus Biologicals, part of Bio-Techne | Catalog # NBP2-52986

Key Product Details
Species
Bacteria
Applications
Chromatin Immunoprecipitation, Immunocytochemistry/ Immunofluorescence, Immunohistochemistry, Immunoprecipitation, Simple Western, Western Blot
Product Summary for CRISPR-Cas9 Antibody Pack
This pack contains 1 vial each of: NBP2-36440SS (0.025 mL), NBP2-52398SS (0.025 mL).
Product Specifications
Application Notes
See individual datasheets of components for their validated applications
Reactivity Notes
See individual datasheets of components for their validated species
Scientific Data Images for CRISPR-Cas9 Antibody Pack
Immunocytochemistry/ Immunofluorescence: CRISPR-Cas9 Antibody Pack [NBP2-52986]
Immunocytochemistry/Immunofluorescence: CRISPR-Cas9 Antibody Pack [NBP2-52986] - Hela cells or Hela cells expressing Flag-tagged SpCas9 under the control of the PTight (Tet-ON) promoter were treated for 24h with 1ug/ul Doxycyclin, fixed and permeabilized with Methanol/Acetone and blocked in 2% BSA in PBS for 2 hours at RT. Cells were stained with 6G12 hybridoma supernatant (diluted 1:10) at 4C o/n, followed by incubation with anti mouse-AF488 coupled secondary antibody for 1 h at RT. Nuclei were counter-stained with Hoechst 33342.Western Blot: CRISPR-Cas9 Antibody Pack [NBP2-52986]
Western Blot: CRISPR-Cas9 Antibody Pack [NBP2-52986] - Hela cells and Hela cells expressing Flag-tagged S.pyogenes Cas9 under the control of the PTight (Tet-ON) promoter were treated for 24h with 1ug/ul Doxycyclin and lysed under native conditions. 30ug of whole cell lysate per lane was separated by 7.5% SDS-PAGE, transfered to nitrocellulose membrane and incubated with 6G12 hybridoma supernatant (diluted 1:100) at 4C o/n. After washing, the membranes were incubated with secondary HRP-coupled antibody and bands were visualized by ECL and exposure of X-ray films. Prestained marker bands were visualized with Blue Marker Antibody (NBP2-33376). A 1 min exposure is shown. Note the specific band of CRISPR-Cas9 with a molecular weight of 158.4 kDa.
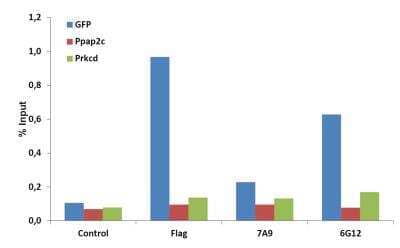
Chromatin Immunoprecipitation (ChIP): CRISPR-Cas9 Antibody Pack [NBP2-52986] - ChIP analysis of CRISPR-Cas9 antibody clones 7A9-3A3 and 6G12 on NIH3T3 cells which were stably expressing GFP-H2B, nuclease dead Cas9 (Flag tagged), and a GFP-targeting gRNA. The cells were fixed with formaldehyde, harvested and sonicated to get 200-500bp DNA fragments. 50ug chromatin was incubated over night at 4C with the indicated antibodies (200ul hybridoma supernatants of clones 7A9 and 6G12; 5ug Flag antibody) followed by incubation with protein G beads for 3h at 4C. After washing chromatin was eluted from the beads and crosslinking was reversed over night at 65C. After a proteinase K digestion step, DNA was separated using phenol/chloroform/isoamyl alcohol, precipitated with ethanol/sodium acetate and dissolved in water. For qPCR, primers either targeting the GFP gene or as negative control non-targeted regions (Ppap2c +7122 and Prkcd +24069 from transcription start) were used.
Kit Contents for CRISPR-Cas9 Antibody Pack
Formulation, Preparation, and Storage
Concentration
Concentration of individual antibodies may be found on the vial label. If unlisted please contact technical services.
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below.
Storage
Store at 4C short term. Aliquot and store at -20C long term. Avoid freeze-thaw cycles.
Background: CRISPR-Cas9
Using CRISPR-Cas9 technology, double-stranded DNA breaks may be induced within specific targeted genome sequences (target DNA; protospacer) for insertion or removal of DNA sequences for gene editing applications. To target a specific loci, a gRNA that will bind to a specific target sequence of DNA within a genome is created. The gRNA will recognize the DNA sequence, and the Cas9 enzyme will cleave the DNA at the targeted location. Once the targeted DNA is removed by Cas9, the cell's own DNA repair mechanism is used to insert or remove a DNA sequence for genomic editing.
Cas9 detection is used to confirm and evaluate CRISPR Cas9 gRNA transfection efficiency. Western blot analysis of CRISPR-Cas9 gRNA transfected cell lysates with Cas9 antibodies identifies the protein having a theoretical molecular weight of 160kDa. Broad areas of research are benefiting from CRISPR-Cas9 based gene editing tools including studies of basic immunity functions, genetic screening and disease treatment (2). Ethical concerns have led to many countries making it illegal to manipulate human germline cells or perform embryo genome editing.
References
1. Oakes, B. L., Fellmann, C., Rishi, H., Taylor, K. L., Ren, S. M., Nadler, D. C., . . . Savage, D. F. (2019). CRISPR-Cas9 Circular Permutants as Programmable Scaffolds for Genome Modification. Cell, 176(1-2), 254-267.e216. doi:10.1016/j.cell.2018.11.052
2. Chiou, S. H., Winters, I. P., Wang, J., Naranjo, S., Dudgeon, C., Tamburini, F. B., . . . Winslow, M. M. (2015). Pancreatic cancer modeling using retrograde viral vector delivery and in vivo CRISPR/Cas9-mediated somatic genome editing. Genes Dev, 29(14), 1576-1585. doi:10.1101/gad.264861.115
Long Name
CRISPR-associated Protein 9
Alternate Names
Cas9, CRISPR-associated endonuclease Cas9/Csn1, CRISPR-Cas9/Csn1, CRISPR/Cas9, csn1, SPy_1046, SPy1046, SpyCas9, CRISPR-associated protein 9 nuclease
Additional CRISPR-Cas9 Products
Product Specific Notices for CRISPR-Cas9 Antibody Pack
This product is for research use only and is not approved for use in humans or in clinical diagnosis. Antibody Packs are guaranteed for 1 year from date of receipt.
Loading...
Loading...
Loading...
Loading...
![Western Blot: CRISPR-Cas9 Antibody Pack [NBP2-52986] Western Blot: CRISPR-Cas9 Antibody Pack [NBP2-52986]](https://resources.bio-techne.com/images/products/CRISPR-Cas9-Antibody-Pack-Western-Blot-NBP2-52986-img0005.jpg)
![Western Blot: CRISPR-Cas9 Antibody Pack [NBP2-52986] Western Blot: CRISPR-Cas9 Antibody Pack [NBP2-52986]](https://resources.bio-techne.com/images/products/CRISPR-Cas9-Antibody-Pack-Western-Blot-NBP2-52986-img0003.jpg)
![Immunoprecipitation: CRISPR-Cas9 Antibody Pack [NBP2-52986] Immunoprecipitation: CRISPR-Cas9 Antibody Pack [NBP2-52986]](https://resources.bio-techne.com/images/products/CRISPR-Cas9-Antibody-Pack-Immunoprecipitation-NBP2-52986-img0002.jpg)
![Immunocytochemistry/ Immunofluorescence: CRISPR-Cas9 Antibody Pack [NBP2-52986] Immunocytochemistry/ Immunofluorescence: CRISPR-Cas9 Antibody Pack [NBP2-52986]](https://resources.bio-techne.com/images/products/CRISPR-Cas9-Antibody-Pack-Immunocytochemistry-Immunofluorescence-NBP2-52986-img0004.jpg)