Human M-CSF Antibody
R&D Systems, part of Bio-Techne | Catalog # AB-216-NA

Key Product Details
Validated by
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
Glu33-Ser190
Accession # NP_757350
Specificity
Clonality
Host
Isotype
Endotoxin Level
Scientific Data Images for Human M-CSF Antibody
Cell Proliferation Induced by M-CSF and Neutralization by Human M-CSF Antibody.
Recombinant Human M-CSF (Catalog # 216-MC) stimulates proliferation in the M-NFS-60 mouse myelogenous leukemia lymphoblast cell line in a dose-dependent manner (orange line). Proliferation elicited by Recombinant Human M-CSF (2.5 ng/mL) is neutralized (green line) by increasing concentrations of Goat Anti-Human M-CSF Polyclonal Antibody (Catalog # AB-216-NA). The ND50 is typically 0.05-0.15 µg/mL.Detection of Human M-CSF by Block/Neutralize
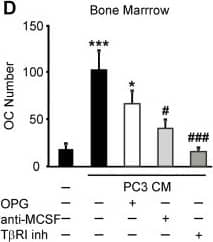
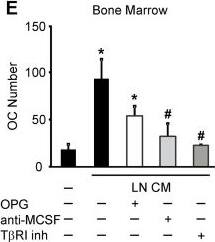
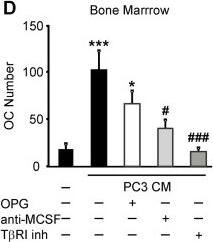
Soluble factors produced by prostate cancer cells increase osteoclast formation in RANKL-independent manner. A, B) RAW 264.7 cells were primed with RANKL for 2 days, then cultured for 2 days untreated (negative control), with RANKL (positive control) or exposed to 10% PC3 or LNCaP CM, in the absence (black bars) or presence of 500 ng/ml OPG (white bars), and the average number of osteoclasts was assessed. A) Representative images of osteoclasts induced by RANKL or prostate cancer CM in the absence (top), or presence (bottom) of OPG. B) Average number of osteoclasts formed in different conditions. Data are means ± SEM; n = 4-10 experiments, except for RANKL and OPG, where n = 2 repeats. C-E) Bone marrow cells were primed with RANKL for 3 days, and cultured for 2 days untreated (negative control), treated with RANKL (positive control) (C) or exposed to 10% CM of PC3 (D) or LNCaP (E) cells, in the absence (black bars), or presence of 500 ng/ml OPG (white bars), or 5 μg/ml anti-MCSF blocking antibody (light gray bars) or T betaRI inhibitor (5 μM, dark gray bars) and the average osteoclast numbers were assessed. OPG and anti-MCSF were added to prostate cancer CM for 30–60 min prior to addition to osteoclast precursors, T betaRI inhibitor was added to the osteoclast precursor cultures for 60 min prior to addition of prostate cancer CM. Data are means ± SEM; n = 3-7 experiments. *P < 0.05, ***P < 0.001 indicate significance compared to negative control; #P < 0.05, ###P < 0.005 indicate significance as compared to no inhibitor as assessed by Student’s t-test, no significant difference between samples treated with CM with and without OPG. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/24370273), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of Human M-CSF by Block/Neutralize
Soluble factors produced by prostate cancer cells increase osteoclast formation in RANKL-independent manner. A, B) RAW 264.7 cells were primed with RANKL for 2 days, then cultured for 2 days untreated (negative control), with RANKL (positive control) or exposed to 10% PC3 or LNCaP CM, in the absence (black bars) or presence of 500 ng/ml OPG (white bars), and the average number of osteoclasts was assessed. A) Representative images of osteoclasts induced by RANKL or prostate cancer CM in the absence (top), or presence (bottom) of OPG. B) Average number of osteoclasts formed in different conditions. Data are means ± SEM; n = 4-10 experiments, except for RANKL and OPG, where n = 2 repeats. C-E) Bone marrow cells were primed with RANKL for 3 days, and cultured for 2 days untreated (negative control), treated with RANKL (positive control) (C) or exposed to 10% CM of PC3 (D) or LNCaP (E) cells, in the absence (black bars), or presence of 500 ng/ml OPG (white bars), or 5 μg/ml anti-MCSF blocking antibody (light gray bars) or T betaRI inhibitor (5 μM, dark gray bars) and the average osteoclast numbers were assessed. OPG and anti-MCSF were added to prostate cancer CM for 30–60 min prior to addition to osteoclast precursors, T betaRI inhibitor was added to the osteoclast precursor cultures for 60 min prior to addition of prostate cancer CM. Data are means ± SEM; n = 3-7 experiments. *P < 0.05, ***P < 0.001 indicate significance compared to negative control; #P < 0.05, ###P < 0.005 indicate significance as compared to no inhibitor as assessed by Student’s t-test, no significant difference between samples treated with CM with and without OPG. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/24370273), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Human M-CSF Antibody
Western Blot
Sample: Recombinant Human M-CSF (Catalog # 216-MC)
Neutralization
Formulation, Preparation, and Storage
Purification
Reconstitution
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: M-CSF
M-CSF, also known as CSF-1, is a four-alpha -helical-bundle cytokine that is the primary regulator of macrophage survival, proliferation and differentiation (1‑3). M-CSF is also essential for the survival and proliferation of osteoclast progenitors (1, 4). M-CSF also primes and enhances macrophage killing of tumor cells and microorganisms, regulates the release of cytokines and other inflammatory modulators from macrophages, and stimulates pinocytosis (2, 3). M-CSF increases during pregnancy to support implantation and growth of the decidua and placenta (5). Sources of M-CSF include fibroblasts, activated macrophages, endometrial secretory epithelium, bone marrow stromal cells and activated endothelial cells (1‑5). The M-CSF receptor (c-fms) transduces its pleotropic effects and mediates its endocytosis. M-CSF mRNAs of various sizes occur (3‑9). Full length human M-CSF transcripts encode a 522 amino acid (aa) type I transmembrane (TM) protein with a 464 aa extracellular region, a 21 aa TM domain, and a 37 aa cytoplasmic tail that forms a 140 kDa covalent dimer. Differential processing produces two proteolytically cleaved, secreted dimers. One is an N- and O- glycosylated 86 kDa dimer, while the other is modified by both glycosylation and chondroitin-sulfate proteoglycan (PG) to generate a 200 kDa subunit. Although PG-modified M-CSF can circulate, it may be immobilized by attachment to type V collagen (8). Shorter transcripts encode M‑CSF that lacks cleavage and PG sites and produces an N-glycosylated 68 kDa TM dimer and a slowly produced 44 kDa secreted dimer (7). Although forms may vary in activity and half-life, all contain the N-terminal 150 aa portion that is necessary and sufficient for interaction with the M-CSF receptor (10, 11). The first 223 aa of mature human M-CSF shares 88%, 86%, 81% and 74% aa identity with corresponding regions of dog, cow, mouse and rat M-CSF, respectively (12, 13). Human M‑CSF is active in the mouse, but mouse M-CSF is reported to be species-specific.
References
- Pixley, F.J. and E.R. Stanley (2004) Trends Cell Biol. 14:628.
- Chitu, V. and E.R. Stanley (2006) Curr. Opin. Immunol. 18:39.
- Fixe, P. and V. Praloran (1997) Eur. Cytokine Netw. 8:125.
- Ryan, G.R. et al. (2001) Blood 98:74.
- Makrigiannakis, A. et al. (2006) Trends Endocrinol. Metab. 17:178.
- Nandi, S. et al. (2006) Blood 107:786.
- Rettenmier, C.W. and M.F. Roussel (1988) Mol. Cell Biol. 8:5026.
- Suzu, S. et al. (1992) J. Biol. Chem. 267:16812.
- Manos, M.M. (1988) Mol. Cell. Biol. 8:5035.
- Koths, K. (1997) Mol. Reprod. Dev. 46:31.
- Jang, M-H. et al. (2006) J. Immunol. 177:4055.
- Kawasaki, E.S. et al. (1985) Science 230: 291.
- Wong, G.G. et al. (1987) Science 235:1504.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional M-CSF Products
Product Documents for Human M-CSF Antibody
Product Specific Notices for Human M-CSF Antibody
For research use only