Human PSGL-1/CD162 Antibody
R&D Systems, part of Bio-Techne | Catalog # MAB996

Key Product Details
Validated by
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
Specificity
Clonality
Host
Isotype
Endotoxin Level
Scientific Data Images for Human PSGL-1/CD162 Antibody
Detection of Human PSGL-1/CD162 by Flow Cytometry
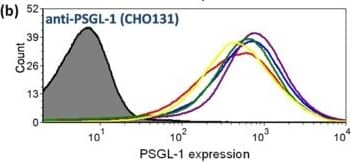
Flow cytometric analysis reveals bromelain cleaves PSGL-1 within its active site in a dose-dependent manner.To analyze neutrophil expression of PSGL-1, the primary ligand for P-selectin, we used two anti-PSGL-1 antibodies that recognize distinct structural motifs within the PSGL-1 active site. (a) Representative data from an analysis with clone KPL-1 reveals an 80% decrease in PSGL-1 expression following treatment with 100 µg/mL bromelain. (b) Representative data from an analysis with clone CHO131 reveals a slight increase followed by a decrease in PSGL-1 expression back to initial levels as bromelain concentrations increase from 0 to 100 µg/mL. (c) The average PSGL-1 expression levels of neutrophils from n = 3–4 donors (±SEM) plotted as a function of bromelain concentration, suggesting that bromelain cleaves PSGL-1 at a position between the two epitopes recognized by the site-specific antibodies. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24244398), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Human PSGL-1/CD162 Antibody
Adhesion Blockade
CyTOF-ready
Flow Cytometry
Sample: Human whole blood monocytes and granulocytes
Formulation, Preparation, and Storage
Purification
Reconstitution
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: PSGL-1/CD162
Human PSGL-1 (P-Selectin Glycoprotein Ligand-1; also CD162), is a 120 kDa mucin-type glycoprotein that plays a key role in leukocyte adhesion (1-3). It is synthesized as a 412 amino acid (aa) preproprecursor that contains a 17 aa signal sequence, a 24 aa propeptide, a 279 aa extracellular domain (ECD), a 21 aa transmembrane segment and a 71 aa cytoplasmic region (4, 5). Following cleavage of the pre- and prosegments, it is expressed as a 240 kDa disulfide-linked homodimer. The extreme N-terminus (aa 1-16 of the mature molecule) contains one threonine (aa 16) and three tyrosines (aa 5, 7, and 10) that are involved in ligand binding. The Thr residue allows for O-linked glycosylation in the form of a core-2 structure (GalNAc-Gal) linked in a beta1,6 bond to a sialylated Lewis X motif (GlcNAc linked to both Fuc and Gal with a terminal sialic acid residue) (1, 2, 5, 6, 7). The three tyrosine residues allow for sulfation (8, 9). When binding to P-selectin, Tyr sulfation and glycosylation are essential. Tyr7 provides the most efficient sulfate moiety, while Fuc and sialic acid are essentially mandatory (7). When binding to E‑selectin, only carbohydrate is needed, while both carbohydrate and Tyr10 are used for L-selectin binding (6, 8). There are 16 decameric aa repeats in the ECD of the longform of PSGL-1. This form is referred to as the A allele, and represents 65 - 80% of the population. Alleles B and C show deletions of decameric repeats #2 (aa 132‑141) plus #9 and 10 (aa 222-241), respectively. Shorter forms may show weaker binding to P-selectin (9, 10). Soluble forms of PSGL-1 are also known. Neutrophil elastase will cleave somewhere within repeats #5-9, while cathepsin G cleaves after Tyr7 (11). The loss of Tyr5 and 7 should impact binding affinity. PSGL‑1 is found on virtually all leukocytes and macrophages/DC’s (1). Although there is similarity in the organization of the ECD between species, there is little aa identity. Human PSGL-1 ECD shares 51%, 52% and 43% aa sequence identity with equine, canine and mouse ECD, respectively.
References
- Yang, J. et al. (1999) Thromb. Haemost. 81:1.
- Cummings, R.D. (1999) Braz. J. Med. Biol. Res. 32:519.
- McEver, R.P. and R.D. Cummings (1997) J. Clin. Invest. 100:485.
- Sako, D. et al. (1993) Cell 75:1179.
- Veldman, G.M. et al. (1995) J. Biol. Chem. 270:16470.
- Bernimoulin, M.P. et al. (2003) J. Biol. Chem. 278:37.
- Leppanen, A. et al. (2000) J. Biol. Chem. 275:39569.
- Sako, D. et al. (1995) Cell 83:323.
- Afshar-Kharghan, V. et al. (2001) Blood 97:3306.
- Lozano, M.L. et al. (2001) Br. J. Haematol. 115:969.
- Gardiner, E.E. et al. (2001) Blood 98:1440.
Long Name
Alternate Names
Gene Symbol
Additional PSGL-1/CD162 Products
Product Documents for Human PSGL-1/CD162 Antibody
Product Specific Notices for Human PSGL-1/CD162 Antibody
For research use only