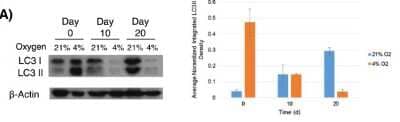
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Autophagy mobilized during differentiation of immortalized human mesenchymal stem cells (ihMSCs). Cultured ihMSCs were stimulated to undergo osteogenesis to form osteoblasts for 30 days at 21% and 4% oxygen. LC3I and LC3II levels were assessed via immunoblot every 10 days and normalized to beta-actin to derive average LC3II band densities. Image collected and cropped by CiteAb from the following publication (//pubmed.ncbi.nlm.nih.gov/25523618/) licensed under a CC-BY license.
Western Blot: LC3A AntibodyBSA Free [NB100-2331]
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody [NB100-2331] - Autophagy mobilized during differentiation of immortalized human mesenchymal stem cells (ihMSCs). Cultured ihMSCs were stimulated to undergo osteogenesis to form osteoblasts for 30 days at 21% and 4% oxygen. LC3I and LC3II levels were assessed via immunoblot every 10 days and normalized to beta-actin to derive average LC3II band densities. Image collected and cropped by CiteAb from the following publication (https://stemcellres.com/content/5/6/140), licensed under a CC-BY license.
Western Blot: LC3A AntibodyBSA Free [NB100-2331]
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody [NB100-2331] - This LC3A antibody Image shows Analysis in human cell lysates.
Western Blot: LC3A AntibodyBSA Free [NB100-2331]
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody [NB100-2331] - This LC3A antibody Image shows analysis of heart tissue lysates from mice which were subjected or not to 48 hours of starvation. The signal was developed using ECL method and this LC3 antibody was found to detect both forms of LC3, i.e. LC3A and LC3B. As expected, the levels of LC3B form were higher in the heart tissue lysates from starved mice.
Western Blot: LC3A AntibodyBSA Free [NB100-2331]
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody [NB100-2331] - Detection of HRP conjugated autophagic LC3 in mouse ES cell lysate. The atg5-/- lane (ES cells, cultured to form embryonic bodies, that are deficient in conversion of LC3-1 to LC3-11) demonstrates the specificity of NB 100-2331, as there is no detection of LC3-11. Photo courtesy of Dr. Beth Levine, UT Southwestern Medical Center.
Immunocytochemistry/ Immunofluorescence: LC3A Antibody - BSA Free [NB100-2331]
Immunocytochemistry/Immunofluorescence: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody [NB100-2331] - This LC3A antibody Image shows an analysis in HeLa cells using anti-LC3 antibody (red). Nuclei were counterstained with DAPI (blue).
Immunocytochemistry/ Immunofluorescence: LC3A Antibody - BSA Free [NB100-2331]
Immunocytochemistry/Immunofluorescence: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody [NB100-2331] - Left panel shows untreated HeLa cells. Right panel shows HeLa cells that were treated with 50 uM CQ overnight. Cells were fixed for 10 minutes using 10% formalin and then permeabilized for 5 minutes using 1X PBS + 0.05% Triton X-100. The cells were incubated with anti-LC3A antibody at 5 ug/mL overnight at 4C and detected with an anti-mouse DyLight 488 (Green) at a 1:500. Nuclei were counterstained with DAPI (Blue). Cells were imaged using a 40X objective.
Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331]
Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody [NB100-2331] - Rapamycin increases autophagy in brains of PDAPP mice. Representative epifluorescent (c200x) image of hippocampal CA1 in control- and rapamycin-fed transgenic PDAPP mice stained with an anti-LC3 antibody. An increase in LC3-immunoreactive puncta was observed in CA1 projections of transgenic PDAPP mice following rapamycin administration. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0009979) licensed under a CC-BY license.
Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331]
Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody [NB100-2331] - Analysis in mouse renal tissue. Image from verifed customer review.
Simple Western: LC3A AntibodyBSA Free [NB100-2331]
Simple Western: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody [NB100-2331] - Image shows a specific band for LC3 in 0.5 mg/mL of Neuro2A lysate. This experiment was performed under reducing conditions using the 12-230 kDa separation system.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Chronic, third window RIC increases the expression of autophagosome proteins, LC3I/II & Atg5.(A) Western blots for autophagy related signaling proteins. (B) Quantification of the protein fold change in 3W RIC compared to 3W controls. Values are means ± S.E.M. n = 6–8 per group. An (*) denotes a statistically significant difference (P<0.05) compared to control. (P-: phospho-). Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25347774), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - PRDX3 expression & its association with autophagy flux in cultured prostate cells(A) Representative immunoblot showing the levels of PRDX3, TOM20 & LC3-II in in lysates prepared from three different cultures of BPH-1 & RWPE-1 cells in the absence (Ctrl) or presence of bafilomycin A1 (BAF). (B–D) The quantification of the relative levels of PRDX3 (B), TOM20 (C) & LC3-II (D) to beta-Actin as shown in (A). Data are mean & standard deviation of three repeats & differences are tested with Student's T-test. *P ≤ 0.05; **P ≤ 0.01. Image collected & cropped by CiteAb from the following publication (https://www.oncotarget.com/lookup/doi/10.18632/oncotarget.17927), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Acute, first window RIC activates autophagy signaling via p-AMPK upregulation & concomitant downregulation of mTOR.(A) Western blots for autophagy related signaling proteins. (B) Quantification of the protein fold change in 1W RIC compared to 1W controls. Values are means ± S.E.M. n = 6–8 per group. An (*) denotes a statistically significant difference (P<0.05) compared to control. (P-: phospho-). Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25347774), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LiCl administered via 0.5% LiCl food pellets for 4 wks does not increase markers for autophagy in Gfap-R236H/+ mice.Each lane of the immunoblots is tissue from one mouse. Immunoblots for LC3-I & LC3-II in (A) did not detect a change in parietal cortex (and underlying white matter) with LiCl treatment. LC3-II bands normalized to LC3-I are quantified in B (N = 3–4 mice from 3–4 cages per genotype, & is representative of 3 blots). LC3-II normalized to GAPDH gave similar results & is not shown. P62 was increased in control diet R236H/+ mouse olfactory bulb compared with control diet +/+, but LiCl did not change P62 levels in GFAP+/+ or R236H/+ mice (C-D). P62 was normalized to total protein loaded. Error bars are SEM. ****P < 0.0001. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26378915), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - MIR376A overexpression blocked autophagy in Huh-7 cells.(A) MIR376A blocked GFP-LC3 dot formation under starvation condition. (B) Quantitative analysis of experiments in A (mean ± S.D. of independent experiments, n = 3, ***p<0,01). (C) Overexpression of MIR376A resulted in decreased autophagic flux in Huh-7 cells. Starvation-induced conversion of LC3-I to LC3-II was analyzed. Tests were performed in the presence or absence of E64d (10 µg/ml) & Pepstatin A (10 µg/ml) (E+P). LC3-II/LC3-I densitometric ratios were marked. ACTB was used as a loading control. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24358205), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] -
Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] - Elevated levels of additional proteins & GM3 ganglioside in the MEC of MPS IIIB brain.Staining performed with antibodies to the indicated substances was observed in the MEC region of 3 month-old MPS IIIB mice (for total ubiquitin & polyubiquitin) & 6 months for all others. Staining was not seen in the MEC region of age-matched control mice (Naglu +/−) nor in the LEC region of MPS IIIB mice (the latter not shown). Image collected & cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0027461), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Autophagy responds rapidly to changing glucose conditions. Immortalized MSCs were cultured in physiologic (also called low in culture parlance; 1 g/L; 5.5 mM) or high (4.5 g/L; 25 mM) glucose media for 2 days & then changed to the corresponding opposite glucose concentration for up to 96 h. Myosin light chain 3 (LC3) levels were probed via immunoblot to assess autophagic response (a). The role of oxygen in the glucose response was also assessed by culturing the MSCs in a Biospherix hypoxic chamber at 4% & 1% oxygen in high & low glucose media for 4 days, followed by comparable LC3 blots (b). Shown are representative blots of three repeated studies. alpha-Actinin was used as a housekeeping control for all blots Image collected & cropped by CiteAb from the following publication (https://stemcellres.biomedcentral.com/articles/10.1186/s13287-016-0436-7), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] -
Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] - Elevated levels of various proteins in MEC & in dentate gyrus of MPS IIIA brain.Staining was performed with antibodies against the proteins shown in a 7 months-old MPS IIIA mouse brain. The first 3 rows are for the MEC region & the bottom row for dentate gyrus. Age-matched C57BL6 mice, used as controls, showed no staining in the MEC region (not shown). The dentate gyrus showed AT270 inclusions in MPS IIIA (−/−) mouse brain (arrows) but not in the C57BL/6 (control) brain (bottom row). Image collected & cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0027461), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Autophagy responds rapidly to changing glucose conditions. Immortalized MSCs were cultured in physiologic (also called low in culture parlance; 1 g/L; 5.5 mM) or high (4.5 g/L; 25 mM) glucose media for 2 days & then changed to the corresponding opposite glucose concentration for up to 96 h. Myosin light chain 3 (LC3) levels were probed via immunoblot to assess autophagic response (a). The role of oxygen in the glucose response was also assessed by culturing the MSCs in a Biospherix hypoxic chamber at 4% & 1% oxygen in high & low glucose media for 4 days, followed by comparable LC3 blots (b). Shown are representative blots of three repeated studies. alpha-Actinin was used as a housekeeping control for all blots Image collected & cropped by CiteAb from the following publication (https://stemcellres.biomedcentral.com/articles/10.1186/s13287-016-0436-7), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Delayed, second window RIC maintains mTOR inhibition without activating autophagosome machinery.(A) Western blots for autophagy related signaling proteins. (B) Quantification of the protein fold change in 2W RIC compared to 2W controls. Values are means ± S.E.M. n = 6–8 per group. An (*) denotes a statistically significant difference (P<0.05) compared to control. (P-: phospho-). Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25347774), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] -
Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] - Elevated levels of various proteins in MEC & in dentate gyrus of MPS IIIA brain.Staining was performed with antibodies against the proteins shown in a 7 months-old MPS IIIA mouse brain. The first 3 rows are for the MEC region & the bottom row for dentate gyrus. Age-matched C57BL6 mice, used as controls, showed no staining in the MEC region (not shown). The dentate gyrus showed AT270 inclusions in MPS IIIA (−/−) mouse brain (arrows) but not in the C57BL/6 (control) brain (bottom row). Image collected & cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0027461), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Modulation of A152T-tau clearance & pathology by upregulation of autophagy. (J–N) Injection of an expression vector encoding zebrafish atg5 into A152T-tau fish embryos resulted in over-expression of Atg5 protein at 2 dpf (J & K) (high & low exposure of same blot presented; mean ± SD, n = 6 independent clutches; two-tailed t-test, *P < 0.05 versus control). (J & L) The increase in Atg5 protein correlated with increase in LC3II, a well-characterized reporter for autophagosome number, demonstrating that autophagy was upregulated in Atg5-injected fish (mean ± SEM, n = 8 independent clutches; two-tailed t-test, ***P < 0.001 versus control). Image collected & cropped by CiteAb from the following publication (https://academic.oup.com/brain/article/140/4/1128/2980948), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] -
Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] - Elevated levels of additional proteins & GM3 ganglioside in the MEC of MPS IIIB brain.Staining performed with antibodies to the indicated substances was observed in the MEC region of 3 month-old MPS IIIB mice (for total ubiquitin & polyubiquitin) & 6 months for all others. Staining was not seen in the MEC region of age-matched control mice (Naglu +/−) nor in the LEC region of MPS IIIB mice (the latter not shown). Image collected & cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0027461), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Impacts of PRDX3 protein on autophagy flux(A–D) Representative immunoblot (A, C) & quantification (B, D) showing the levels of LC3-II in BPH-1 cells treated with random (MOCK) or PRDX3-specific siRNA (PRDX3) (A, B) or RWPE-1 cells transiently expressing different amount of PRDX3 (C, D) in the absence (Ctrl) or presence of bafilomycin A1 (BAF). Data are mean & standard deviation of three repeats & differences are tested with Student's T-test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. (E–G) Representative immunoblot (E) & quantification (F, G) showing the levels of Beclin 1 (F) & PI3KCIII (G) in BPH-1 cells treated with random (MOCK) or PRDX3-specific siRNA (PRDX3). Ns, not significant; *P ≤ 0.05. Image collected & cropped by CiteAb from the following publication (https://www.oncotarget.com/lookup/doi/10.18632/oncotarget.17927), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Endogenous MIR376A limits starvation-induced autophagy.(A) Blockage of endogenous MIR376A by Ant-376a, but not CNT-Ant further stimulated starvation (STV)-activated LC3-I to LC3-II conversion in MCF-7 cells. ACTB was used as a loading control. LC3-II/LC3-I densitometric ratios were marked. (B) Ant-376a, but not CNT-Ant resulted in further activation of SQSTM1 protein degradation following starvation in MCF-7 cells. SQSTM1/ACTB ratios were marked. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24358205), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Dram1 is required for GFP-Lc3 targeting to Mm clusters.a, b Representative confocal micrographs & quantification of GFP-Lc3 puncta in dram1∆19n/∆19n & dram1+/+ larvae in an unstimulated situation (basal autophagy, a) & following BafA1 treatment b. Each larva was imaged at a pre-defined region of the tail fin (≥11 larvae/group). Results are accumulated from two independent experiments & represented by scatter & boxplots as detailed in the “Methods” section. ns non-significant, *p < 0.05,**p < 0.01,***p < 0.001. Scale bars, 10 μm. The intensity calibration bar for the Lookup table (LUT) is displayed in panel a. c–e Western blot analysis of autophagy. Protein samples were obtained from 4 dpf dram1∆19n/∆19n & dram1+/+ larvae (>10 larvae/sample). Lc3 c & e, or p62 & Optineurin d protein levels were detected in absence or presence of BafA1, c & d, or in the presence or absence of Mm e. Actin was used as a loading control. Western Blots were repeated three, c & d, or two e times with protein extracts derived from independent experiments. The Lc3II/Actin or p62/Actin & Optineurin/Actin ratio, normalized to the control sample, is indicated below the blots. f–g Representative confocal micrographs & quantification of GFP-Lc3 co-localization with Mm clusters in infected dram1∆19n/∆19n & dram1+/+ larvae. The top images f show the entire region of imaging, while the bottom images f′ & f″ show details of GFP-Lc3 colocalization of Mm clusters in dram1∆19n/∆19n & dram1+/+ larvae. The arrowheads indicate GFP-Lc3-positive Mm clusters. The data is accumulated from two independent experiments (≥15 larvae/group) & represented by scatter & boxplots as detailed in the “Methods” section. Scale bars, 10 μm. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32332700), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Optn or p62 deficiency affects autophagosome formation.(A) Workflow of the experiments shown in (B-G). Larvae were treated with 100 nM of Baf A1 for 12 h from 3.5 dpf. The GPF-Lc3 negative larvae were selected to assay autophagy activity by Western blot, the GFP-Lc3 positive larvae were collected to monitor autophagic activity using confocal imaging. The red square indicates the region for confocal imaging. (B) Level of basal autophagy in WT & mutant embryos in absence or presence of Baf A1. Protein samples were extracted from 4 dpf WT & mutant larvae (>10 embryos/sample). The blots were probed with antibodies against Lc3 & Actin as a loading control. Western blots were repeated at least three times with independent extracts. (C) Quantification of Lc3-II fold changes in WT & mutant embryos in absence or presence of Baf A1. Western blot band intensities were quantified by Lab Image. Data is combined from three independent experiments. (D) Representative confocal micrographs of GFP-Lc3 puncta present in the tail fin of optn+/+, optn delta5n/ delta5n, p62+/+ & p62 delta37n/ delta37n at 4 dpf. Scale bars, 10 μm. (E). Quantification of the number of GFP-Lc3 puncta in optn+/+, optn delta5n/ delta5n, p62+/+ & p62 delta37n/ delta37n larvae with & without Baf A1 treatment. Each larva was imaged at a pre-defined region of the tail fin (as indicated by the red boxed area in Fig3 A) (≥11 larvae/group). Results are accumulated from two independent experiments. ns, non-significant, *p<0.05, **p<0.01, ***p<0.001. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30818338), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] -
Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] - Rapamycin increases autophagy in brains of PDAPP mice.a, f & h, representative immunoblots of hippocampal lysates from control- & rapamycin-treated transgenic PDAPP mice & non-transgenic littermate controls. b, g & i, quantitative analyses. a & b, LC3-II levels are decreased in hippocampi of rapamycin-treated transgenic PDAPP mice (*, P = 0.0009), but not in hippocampi of rapamycin-treated non-transgenic littermates. c & d, representative epifluorescent (c, 200×) & higher-magnification confocal (d, 600×) images of hippocampal CA1 (e, green box, region of epifluorescent images; blue box, region of confocal images) in control- & rapamycin-fed transgenic PDAPP mice stained with an anti-LC3 antibody. An increase in LC3-immunoreactive puncta was observed in CA1 projections of transgenic PDAPP mice following rapamycin administration. f & g, levels of the autophagic substrate p62SQSTM are decreased (*, P = 0.0015) in hippocampi of rapamycin-treated PDAPP transgenic mice. f, representative Western blots; g, quantitative analyses of p62SQSTM levels. h & i, Levels of phosphorylated (activated) p70 were decreased in brains of rapamycin-treated PDAPP & non-transgenic mice (*, P = 0.001 & P = 0.04 respectively). Significance of differences between group means were determined using two-tailed unpaired Student's t test. Data are means ± SEM. Image collected & cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0009979), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Immunocytochemistry/ Immunofluorescence: LC3A Antibody - BSA Free [NB100-2331] -
Immunocytochemistry/ Immunofluorescence: LC3A Antibody - BSA Free [NB100-2331] - Macroautophagy is a major mechanism in the rapid disposal of insulin precursor in beta-cells.The Ins2+/+ beta-cells were cultured under the 5.5 mM glucose concentration for a 24-hour pre-experimental period until treatment. (A) The Ins2+/+ beta-cells were treated with cycloheximide (Chx; 100 µg/mL), Chx (100 µg/mL) & 3-MA (5 mM), Baf A1 (5 µM), or chloroquine (Chl; 100 µg/mL) for 30 minutes with an untreated control. Cellular proteins (30 µg) were separated by 16.5% non-reduced (upper panels) or reduced (lower panels) tricine SDS-PAGE & then examined by immunoblotting. (B) The upper panel, the immunoreactive LC3-I/II (%) in individual treatments in (A); the lower panel, the percentages of proinsulin levels on reduced gels (that were normalized by tubulin) in individual treatments compared to the untreated controls. (C) The Ins2+/+ beta-cells subjected to the same treatments described in (A) were immunostained with antibodies against LC3, C-peptide, & insulin as described in the Materials & Methods. Fluorescent Cy2 (for LC3), Cy3 (for C-peptide & insulin), & their merged images were shown. The scale of bar in (C), 10 µm. (D) The relative levels (%) of the LC3 & (pro)insulin and/or C-peptide positive dots per cell of individual treatments in (C). The data in (B) or (D) were reported as mean ± SD. *P<0.05; **P<0.01, n = 4. Image collected & cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0027647), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - DBA mutations induce autophagy.(A) Immunofluorescence with LC3 antibodies in LCLs derived from a normal control or DBA patients. Higher magnifications are represented in the lower panel. Arrows denote puncta indicative of LC3 recruitment to autophagosomes, or accumulation in autolysosomes. Size bars = 10 µM. (B) Quantification of the percent of cells revealing LC3 puncta compared to the total number of cells in the 60x shots. (C) Western blot analysis of LC3 in DBA LCLs compared to normal controls. The LC3II/actin ratio is determined by densitometer analysis. (D) Representative western blot analysis of p62 levels in normal control & DBA patient LCLs. (E) Densitometer analysis of p62 protein expression from western blots (N = 3) represented in (D). (F) Immunofluorescence with p62 antibodies of LCLs derived from a normal control or DBA patients. Size bars = 10 µM. (G) ImageJ measurements of p62 expression in (F) per total cell area. (H) Representative electron micrographs of LCLs derived from a normal control & RPS17 cells. Control cells have small typically dense lysosomes (*). The much larger autolysosomes (A) are only detected in RPS17 LCLs. The boxed area in the upper right panel is shown at higher magnification in the lower right panel. N = nucleus, ECS = extracellular space. Bars in top panels = 1 µM, bottom panels = 200 nM. Image collected & cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pgen.1004371), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - UBC9 is degraded by autophagy in epithelial cells.(A) Immunolocalization of UBC9 in ultrathin sections of HKs transduced with empty or HPV16 E6/E7 vectors. Original view (left) & higher magnification (right) of boxed regions. Gold particles are selectively enriched in autophagic structures highlighted by arrows. Scale bar = 1 μm. (B) Top: Representative WB of HaCaT cells treated with the indicated autophagic activators (left) & inhibitors (right). Activation of autophagy was monitored by the conversion of LC3 (LC3-I) to the lipidated LC3 (LC3-II) form, a marker of autophagosome production induced by autophagic stimuli [41]. LC3-II accumulation was used to verify autophagic impairment [41]. Bottom: normalized UBC9 expression. Data are expressed as fold over untreated cells. Bars represent means ± SEM of n = 4 different biological replicates. ns: not significant (Kruskal–Wallis one-way ANOVA with Dunn’s post hoc test) compared to vehicle control groups. (C) Representative WB analysis of U-2 OS or MCF7 cells treated with chloroquine. n = 3 different biological replicates. (D) Representative WB analysis of MCF7 cells transduced with scramble or ATG5 shRNA. The observed ATG5 band represents the ATG5-ATG12 conjugated form. LC3-I accumulation is reported to evidence autophagic deficiencies promoted by shATG5. n = 3 replicates of a single transduction. Image collected & cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.ppat.1006262), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Macroautophagy is a major mechanism in the rapid disposal of insulin precursor in beta-cells.The Ins2+/+ beta-cells were cultured under the 5.5 mM glucose concentration for a 24-hour pre-experimental period until treatment. (A) The Ins2+/+ beta-cells were treated with cycloheximide (Chx; 100 µg/mL), Chx (100 µg/mL) & 3-MA (5 mM), Baf A1 (5 µM), or chloroquine (Chl; 100 µg/mL) for 30 minutes with an untreated control. Cellular proteins (30 µg) were separated by 16.5% non-reduced (upper panels) or reduced (lower panels) tricine SDS-PAGE & then examined by immunoblotting. (B) The upper panel, the immunoreactive LC3-I/II (%) in individual treatments in (A); the lower panel, the percentages of proinsulin levels on reduced gels (that were normalized by tubulin) in individual treatments compared to the untreated controls. (C) The Ins2+/+ beta-cells subjected to the same treatments described in (A) were immunostained with antibodies against LC3, C-peptide, & insulin as described in the Materials & Methods. Fluorescent Cy2 (for LC3), Cy3 (for C-peptide & insulin), & their merged images were shown. The scale of bar in (C), 10 µm. (D) The relative levels (%) of the LC3 & (pro)insulin and/or C-peptide positive dots per cell of individual treatments in (C). The data in (B) or (D) were reported as mean ± SD. *P<0.05; **P<0.01, n = 4. Image collected & cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0027647), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Optn or p62 deficiency inhibits targeting of Mm by GFP-Lc3.(A) Workflow of the experiment shown in B. 2 dpi fixed larvae were used for confocal imaging. The entire CHT region was imaged, as indicated by the black box. (B) Representative confocal micrographs of GFP-Lc3 co-localization with Mm clusters in infected larvae. The top image shows an overview of the CHT region in optn+/+ infected larvae. The area indicated by the white box is detailed below. The bottom images show GFP-Lc3 co-localization of Mm clusters in optn+/+, optn delta5n/ delta5n, p62+/+ & p62 delta37n/ delta37n infected larvae. The arrowheads indicate the overlap between GFP-Lc3 & Mm clusters. Scale bars, 10 μm. (C) Quantification of the percentage of Mm clusters positive for GFP-Lc3 vesicles. The data is accumulated from two independent experiments; each dot represents an individual larva (≥12 larvae/group). ns, non-significant, *p<0.05, **p<0.01, ***p<0.001. (D) Western blot analysis of Lc3 protein levels in infected & uninfected larvae. Protein samples were extracted from 4 dpf larvae (>10 larvae/sample). The blots were probed with antibodies against Lc3 & Actin as a loading control & Lc3-II/Lc3-I ratios are indicated below. Western blots were repeated twice with independent extracts. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30818338), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - UBC9 is degraded by autophagy in epithelial cells.(A) Immunolocalization of UBC9 in ultrathin sections of HKs transduced with empty or HPV16 E6/E7 vectors. Original view (left) & higher magnification (right) of boxed regions. Gold particles are selectively enriched in autophagic structures highlighted by arrows. Scale bar = 1 μm. (B) Top: Representative WB of HaCaT cells treated with the indicated autophagic activators (left) & inhibitors (right). Activation of autophagy was monitored by the conversion of LC3 (LC3-I) to the lipidated LC3 (LC3-II) form, a marker of autophagosome production induced by autophagic stimuli [41]. LC3-II accumulation was used to verify autophagic impairment [41]. Bottom: normalized UBC9 expression. Data are expressed as fold over untreated cells. Bars represent means ± SEM of n = 4 different biological replicates. ns: not significant (Kruskal–Wallis one-way ANOVA with Dunn’s post hoc test) compared to vehicle control groups. (C) Representative WB analysis of U-2 OS or MCF7 cells treated with chloroquine. n = 3 different biological replicates. (D) Representative WB analysis of MCF7 cells transduced with scramble or ATG5 shRNA. The observed ATG5 band represents the ATG5-ATG12 conjugated form. LC3-I accumulation is reported to evidence autophagic deficiencies promoted by shATG5. n = 3 replicates of a single transduction. Image collected & cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.ppat.1006262), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Dram1 is required for GFP-Lc3 targeting to Mm clusters.a, b Representative confocal micrographs & quantification of GFP-Lc3 puncta in dram1∆19n/∆19n & dram1+/+ larvae in an unstimulated situation (basal autophagy, a) & following BafA1 treatment b. Each larva was imaged at a pre-defined region of the tail fin (≥11 larvae/group). Results are accumulated from two independent experiments & represented by scatter & boxplots as detailed in the “Methods” section. ns non-significant, *p < 0.05,**p < 0.01,***p < 0.001. Scale bars, 10 μm. The intensity calibration bar for the Lookup table (LUT) is displayed in panel a. c–e Western blot analysis of autophagy. Protein samples were obtained from 4 dpf dram1∆19n/∆19n & dram1+/+ larvae (>10 larvae/sample). Lc3 c & e, or p62 & Optineurin d protein levels were detected in absence or presence of BafA1, c & d, or in the presence or absence of Mm e. Actin was used as a loading control. Western Blots were repeated three, c & d, or two e times with protein extracts derived from independent experiments. The Lc3II/Actin or p62/Actin & Optineurin/Actin ratio, normalized to the control sample, is indicated below the blots. f–g Representative confocal micrographs & quantification of GFP-Lc3 co-localization with Mm clusters in infected dram1∆19n/∆19n & dram1+/+ larvae. The top images f show the entire region of imaging, while the bottom images f′ & f″ show details of GFP-Lc3 colocalization of Mm clusters in dram1∆19n/∆19n & dram1+/+ larvae. The arrowheads indicate GFP-Lc3-positive Mm clusters. The data is accumulated from two independent experiments (≥15 larvae/group) & represented by scatter & boxplots as detailed in the “Methods” section. Scale bars, 10 μm. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32332700), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Knockdown of FUT1 is associated with an increase in autophagic flux. (a) Immunoblot analysis of LC3-II & p62 levels in control & FUT1 knockdown cells. Total cell lysates from MCF-7 & T47D cells transfected with control or FUT1 siRNAs were collected at 120 & 96 h post-transfection, respectively. Equal amounts of cell lysates were then loaded in each lane & separated by SDS-PAGE. Immunoblot analysis was performed with LC3 & p62 antibodies. Actin was used as a loading control. The intensity of LC3-II & p62 protein bands on immunoblot were quantified & normalized to actin, & the relative levels of protein expression were expressed as fold change by setting the control group value to 1. Values shown are mean±S.E.M. of three independent experiments (***P<0.001; **P<0.01; *P<0.05). (b) Downregulation of FUT1 enhanced the fusion of autophagosome & lysosomes in MCF-7 cells. Cells were co-stained with anti-LAMP-1 (green) & anti-LC3 (red) & nuclei stained with Hoechst (blue). Representative colocalization signals (referred to as autolysosomes) were shown in yellow in the merged. Magnification × 63, zoom: × 3. Scale bars, 10 μm. Histogram shows the percentages of autolysosomes (LC3+/LAMP-1+) to autophagosomes (LC3+/LAMP-1−). Data are mean±S.E.M. of three independent experiments of >100 cells per group (*P<0.05) Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27560716), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Effect of MIR376A overexpression on autophagy.(A) MCF-7 cells were co-transfected with either MIR-CNT (control plasmid) or MIR376A & GFP-LC3 plasmid, & GFP-LC3 dot formation was analyzed. White arrows indicate clusters of the GFP-LC3 dots in cells. (B) Quantification of the experiments in A. MIR376A overexpression, but not MIR-CNT expression, blocked starvation (STV)-induced autophagy (mean ± S.D. of independent experiments, n = 4, ***p<0,01). NON STV, non-starved (C) Overexpression of MIR376A resulted in a decrease in the autophagic activity of MCF-7 cells. Starvation-induced conversion of LC3-I to LC3-II in MCF-7 cells was analyzed. Tests were performed in the presence or absence of E64d (10 µg/ml) & Pepstatin A (10 µg/ml) (E+P). LC3-II/LC3-I densitometric ratios were marked. ACTB was used as a loading control. (D) MIR376A blocked starvation induced SQSTM1 degradation in MCF-7 cells. ACTB was used as a loading control. SQSTM1/ACTIN densitometric ratios were marked. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24358205), licensed under a CC-BY license. Not internally tested by Novus Biologicals.
Western Blot: LC3A Antibody - BSA Free [NB100-2331] -
Western Blot: LC3A Antibody - BSA Free [NB100-2331] - Tau clearance in vivo & autophagy function. Clearance kinetics of photoconverted Dendra-tau measured in neurons of WT-tau & AT152T-tau fish. Measurement of intensity of red Dendra-tau signal over time reflects the clearance or degradation of tau protein. (C–F) WBs for LC3-II, a well-characterized marker of autophagosome number, demonstrate that there no differences in levels of this protein between WT-tau & A152T-tau fish either at 24 hpf (pre-phenotype; C & D) or 72 hpf (post-phenotype; E & F). (E & F) Measurements of LC3-II levels in presence or absence of ammonium chloride provides a method for measuring autophagic flux. No differences observed between the two transgenic lines at 3 dpf, suggesting that autophagy functions normally in both WT-tau & A152T-tau fish (graph represents mean ± SD of four independent clutches per group for E & F & 3for C & D; two-tailed t-test). Image collected & cropped by CiteAb from the following publication (https://academic.oup.com/brain/article/140/4/1128/2980948), licensed under a CC-BY license. Not internally tested by Novus Biologicals.

![Western Blot: LC3A AntibodyBSA Free [NB100-2331] Western Blot: LC3A AntibodyBSA Free [NB100-2331]](https://resources.bio-techne.com/images/products/LC3A-Antibody---BSA-Free-Western-Blot-NB100-2331-img0041.jpg)
![Immunocytochemistry/ Immunofluorescence: LC3A Antibody - BSA Free [NB100-2331] Immunocytochemistry/ Immunofluorescence: LC3A Antibody - BSA Free [NB100-2331]](https://resources.bio-techne.com/images/products/LC3A-Antibody---BSA-Free-Immunocytochemistry-Immunofluorescence-NB100-2331-img0046.jpg)
![Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331]](https://resources.bio-techne.com/images/products/LC3A-Antibody---BSA-Free-Immunohistochemistry-NB100-2331-img0045.jpg)
![Western Blot: LC3A AntibodyBSA Free [NB100-2331] Western Blot: LC3A AntibodyBSA Free [NB100-2331]](https://resources.bio-techne.com/images/products/LC3A-Antibody---BSA-Free-Western-Blot-NB100-2331-img0036.jpg)
![Western Blot: LC3A AntibodyBSA Free [NB100-2331] Western Blot: LC3A AntibodyBSA Free [NB100-2331]](https://resources.bio-techne.com/images/products/LC3A-Antibody---BSA-Free-Western-Blot-NB100-2331-img0037.jpg)
![Western Blot: LC3A AntibodyBSA Free [NB100-2331] Western Blot: LC3A AntibodyBSA Free [NB100-2331]](https://resources.bio-techne.com/images/products/LC3A-Antibody---BSA-Free-Western-Blot-NB100-2331-img0039.jpg)
![Western Blot: LC3A AntibodyBSA Free [NB100-2331] Western Blot: LC3A AntibodyBSA Free [NB100-2331]](https://resources.bio-techne.com/images/products/LC3A-Antibody---BSA-Free-Western-Blot-NB100-2331-img0040.jpg)
![Immunocytochemistry/ Immunofluorescence: LC3A Antibody - BSA Free [NB100-2331] Immunocytochemistry/ Immunofluorescence: LC3A Antibody - BSA Free [NB100-2331]](https://resources.bio-techne.com/images/products/LC3A-Antibody---BSA-Free-Immunocytochemistry-Immunofluorescence-NB100-2331-img0038.jpg)
![Immunocytochemistry/ Immunofluorescence: LC3A Antibody - BSA Free [NB100-2331] Immunocytochemistry/ Immunofluorescence: LC3A Antibody - BSA Free [NB100-2331]](https://resources.bio-techne.com/images/products/LC3A-Antibody---BSA-Free-Immunocytochemistry-Immunofluorescence-NB100-2331-img0042.jpg)
![Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331]](https://resources.bio-techne.com/images/products/LC3A-Antibody---BSA-Free-Immunohistochemistry-NB100-2331-img0043.jpg)
![Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331]](https://resources.bio-techne.com/images/products/LC3A-Antibody---BSA-Free-Immunohistochemistry-NB100-2331-img0044.jpg)
![Simple Western: LC3A AntibodyBSA Free [NB100-2331] Simple Western: LC3A AntibodyBSA Free [NB100-2331]](https://resources.bio-techne.com/images/products/LC3A-Antibody---BSA-Free-Simple-Western-NB100-2331-img0025.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415291912.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415334923.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415394531.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415284546.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415384118.jpg)
![Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415382427.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415175261.jpg)
![Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415392587.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-31020241535635.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-31020241517137.jpg)
![Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415334946.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-3102024153436.jpg)
![Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415384175.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415171315.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-31020241534398.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-31020241612632.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415532038.jpg)
![Immunohistochemistry: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-31020241682151.jpg)
![Immunocytochemistry/ Immunofluorescence: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415533836.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415523944.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-31020241682168.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-3102024165730.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415541914.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415525247.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415541920.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-31020241553512.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-310202415535132.jpg)
![Western Blot: LC3A Antibody - BSA Free [NB100-2331] - LC3A Antibody - BSA Free](https://resources.bio-techne.com/images/products/nb100-2331_rabbit-polyclonal-lc3a-antibody-31020241682110.jpg)