Human CXCL10/IP-10 Quantikine QuicKit ELISA Best Seller
R&D Systems, part of Bio-Techne | Catalog # QK266

Key Product Details
Assay Length
Sample Type & Volume Required Per Well
Sensitivity
Assay Range
Product Summary for Human CXCL10/IP-10 Quantikine QuicKit ELISA
Product Specifications
Measurement
Detection Method
Conjugate
Reactivity
Specificity
Cross-reactivity
Interference
Sample Values
| Sample Type | Mean (pg/mL) | Range (pg/mL) | Standard Deviation |
| Serum (n=10) | 85.6 | 49.8-250 | 62.0 |
| EDTA plasma (n=10) | 99.4 | 60.4-205 | 51.8 |
| Heparin plasma (n=10) | 137 | 83.9-281 | 75.0 |
Cell Culture Supernates:
Precision
Intra-Assay Precision (Precision within an assay) Two samples of known concentration were tested twenty times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays) Two samples of known concentration were tested in ten separate assays to assess inter-assay precision.
Cell Culture Supernates, EDTA Plasma, Heparin Plasma, Serum
| Intra-Assay Precision | Inter-Assay Precision | |||
|---|---|---|---|---|
| Sample | 1 | 2 | 1 | 2 |
| n | 20 | 20 | 10 | 10 |
| Mean (pg/mL) | 96.7 | 521 | 95.3 | 515 |
| Standard Deviation | 1.12 | 10.6 | 10.8 | 42.1 |
| CV% | 1.2 | 2.0 | 11.3 | 8.2 |
Recovery for Human CXCL10/IP-10 Quantikine QuicKit ELISA
The recovery of human IP-10 spiked to three levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Media (n=4) | 119 | 110-129 |
| EDTA Plasma (n=2) | 86 | 78-95 |
| Heparin Plasma (n=2) | 104 | 92-118 |
| Serum (n=2) | 71 | 67-76 |
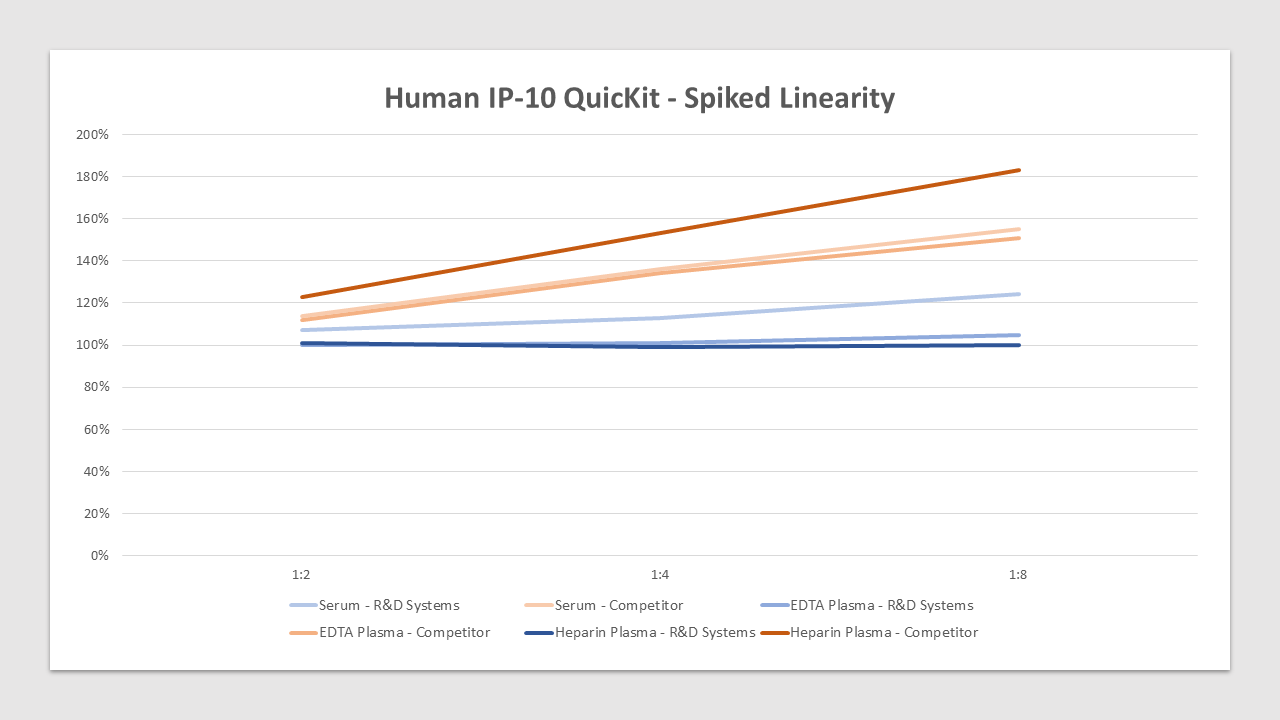
Linearity

Scientific Data Images for Human CXCL10/IP-10 Quantikine QuicKit ELISA
Human IP-10 ELISA Standard Curve
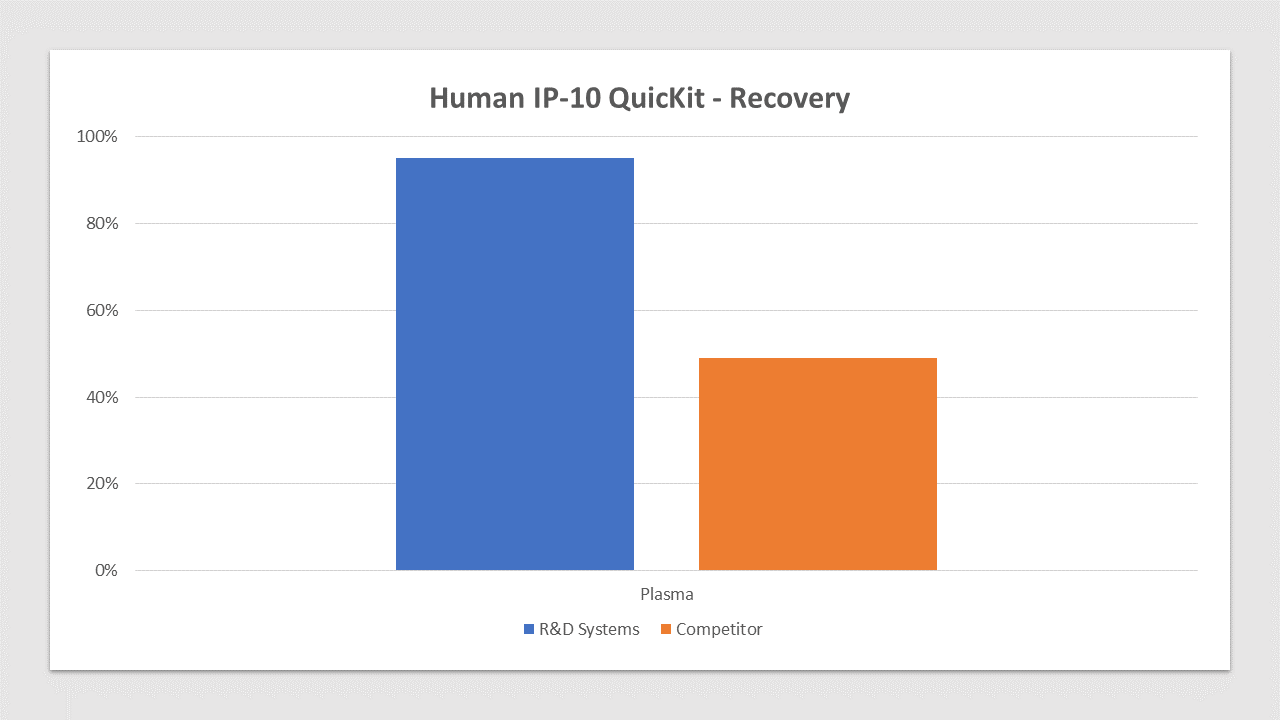
Human IP-10 QuicKit Spiked Recovery Competitor Comparison
IP-10 is spiked at three known concentrations throughout the range of the assay and run to measure response of the spiked sample matrix. Plasma recovery is 95% compared to 49% for the top competitor. In spike and recovery experiments, natural samples are spiked with the recombinant target analyte of interest to identify interference caused by sample matrices.Human IP-10 QuicKit Spiked Linearity Competitor Comparison
IP-10 is spiked at high concentration in various matrices and diluted with appropriate Calibrator Diluent to produce samples with values within the dynamic range of the assay. The linearity is between 99%-124% compared to 112%-183% for the top competitor.Preparation and Storage
Shipping
Stability & Storage
Background: CXCL10/IP-10/CRG-2
Additional CXCL10/IP-10/CRG-2 Products
Product Documents for Human CXCL10/IP-10 Quantikine QuicKit ELISA
Product Specific Notices for Human CXCL10/IP-10 Quantikine QuicKit ELISA
For research use only