Human Erythropoietin/EPO DuoSet ELISA Best Seller
R&D Systems, part of Bio-Techne | Catalog # DY286-05

Key Product Details
Assay Type
Assay Range
Sample Type
Note: Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet
Reactivity
Human Erythropoietin/EPO DuoSet ELISA Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Product Summary for Human Erythropoietin/EPO DuoSet ELISA
Product Specifications
Assay Format
Detection Method
Conjugate
Specificity
Label
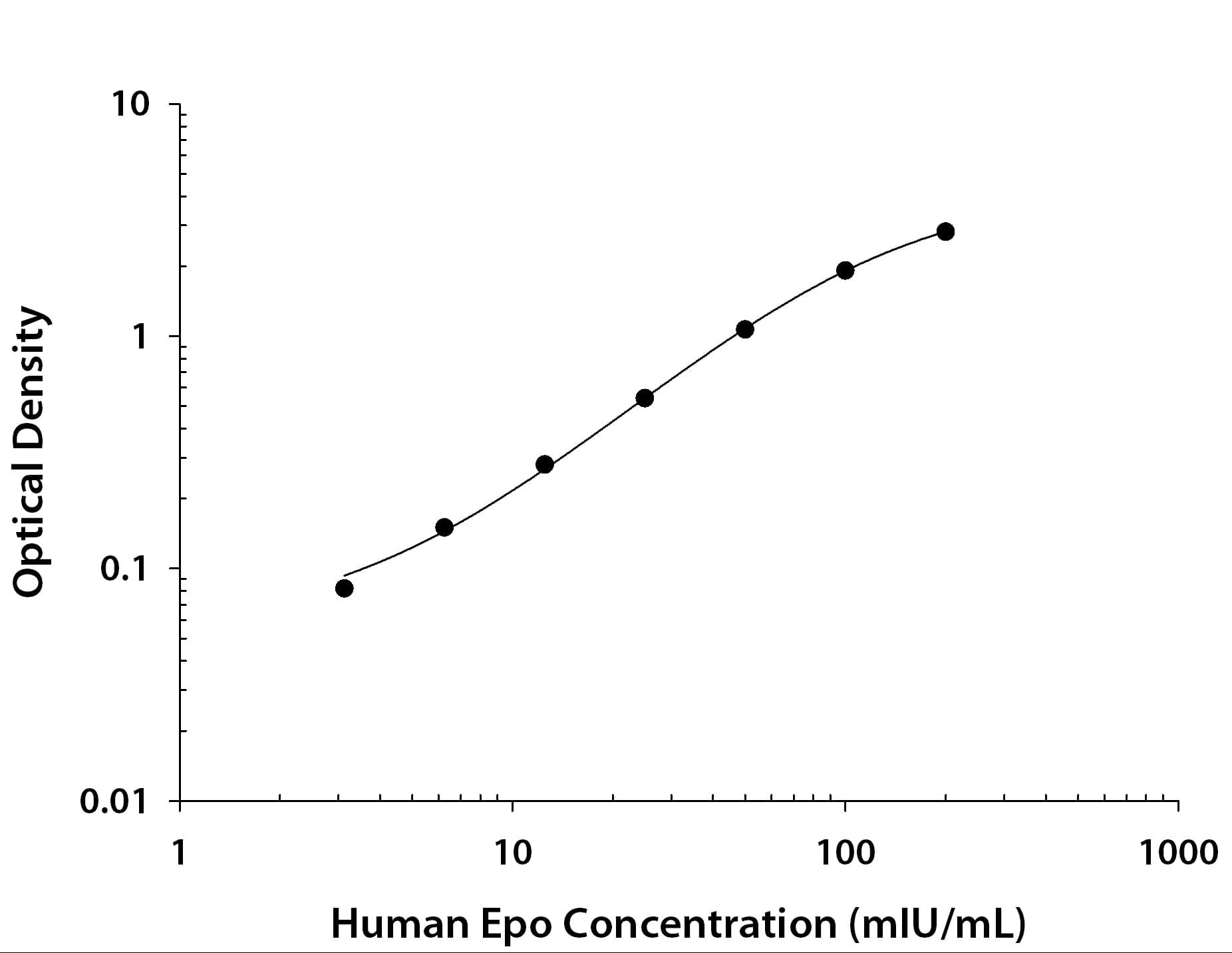
Scientific Data Images for Human Erythropoietin/EPO DuoSet ELISA
Human Erythropoietin ELISA Standard Curve
Kit Contents for Human Erythropoietin/EPO DuoSet ELISA
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Preparation and Storage
Shipping
Stability & Storage
Background: Erythropoietin/EPO
Alternate Names
Gene Symbol
Additional Erythropoietin/EPO Products
Product Documents for Human Erythropoietin/EPO DuoSet ELISA
Product Specific Notices for Human Erythropoietin/EPO DuoSet ELISA
For research use only