Human sTransferrin Receptor/sTfR Quantikine ELISA, RUO
R&D Systems, part of Bio-Techne | Catalog # DTFRU0

Key Product Details
Assay Length
Sample Type & Volume Required Per Well
Sensitivity
Assay Range
Product Summary for Human sTransferrin Receptor/sTfR Quantikine ELISA, RUO
Product Specifications
Measurement
Detection Method
Specificity
Cross-reactivity
Interference
Precision
Intra-Assay Precision (Precision within an assay) Three samples of known concentration were tested twenty times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays) Three samples of known concentration were tested in twenty separate assays to assess inter-assay precision. Assays were performed by at least three technicians using two lots of components.
EDTA Plasma, Heparin Plasma, Serum
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (nmol/L) | 4.82 | 18.4 | 28.8 | 5.02 | 19.8 | 29.5 |
| Standard Deviation | 0.115 | 0.375 | 0.791 | 0.202 | 1.31 | 2.56 |
| CV% | 2.4 | 2.0 | 2.7 | 4.0 | 6.6 | 8.7 |
Recovery for Human sTransferrin Receptor/sTfR Quantikine ELISA, RUO
The recovery of human sTfR spiked to three different levels in samples throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| EDTA Plasma (n=4) | 107 | 98-114 |
| Heparin Plasma (n=4) | 107 | 96-119 |
| Serum (n=4) | 109 | 94-116 |
Linearity
To assess the linearity of the assay, samples spiked with high concentrations of human sTfR in various matrices were diluted with the calibrator diluent to produce samples with values within the dynamic range of the assay.

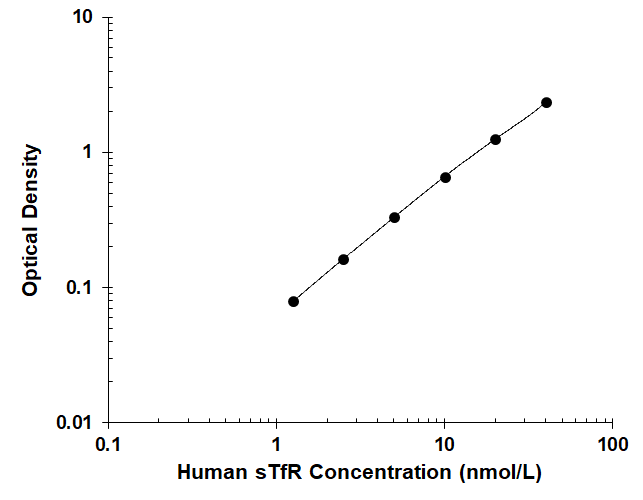
Scientific Data Images for Human sTransferrin Receptor/sTfR Quantikine ELISA, RUO
Human sTfR Quantikine ELISA Standard Curve
Preparation and Storage
Shipping
Stability & Storage
Background: TfR (Transferrin R)
Long Name
Alternate Names
Gene Symbol
Additional TfR (Transferrin R) Products
Product Documents for Human sTransferrin Receptor/sTfR Quantikine ELISA, RUO
Product Specific Notices for Human sTransferrin Receptor/sTfR Quantikine ELISA, RUO
For research use only