Rat IL-2 ELISA Kit (Colorimetric)
Novus Biologicals, part of Bio-Techne | Catalog # NBP1-92704

Key Product Details
Sample Type & Volume Required Per Well
Cell culture supernatant, serum (50 uL)
Sensitivity
11.0 pg/mL
Assay Range
62.5 - 2,000 pg/mL
Product Specifications
Assay Type
Sandwich ELISA
Kit Type
ELISA Kit (Colorimetric)
Reactivity
Rat
Specificity
The interference of circulating factors of the immune system was evaluated by spiking these proteins at physiologically relevant concentrations into a rat IL-2 positive serum. There was no crossreactivity detected.
Description
This kit is an enzyme-linked immunosorbent assay for quantitative detection.
Precision
Intra-Assay Precision (Precision within an assay) CV% < 5.0%
Inter-Assay Precision (Precision between assays) CV% < 10%
Recovery for Rat IL-2 ELISA Kit (Colorimetric)
Recovery
The unspiked serum was used as blank in these experiments.
The overall mean recovery was 81%.
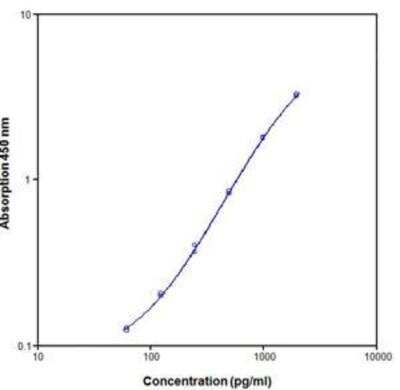
Scientific Data Images for Rat IL-2 ELISA Kit (Colorimetric)
ELISA: Rat IL-2 ELISA Kit (Colorimetric) [NBP1-92704]
Kit Contents for Rat IL-2 ELISA Kit (Colorimetric)
- 1 vial (2.2 ml) monoclonal Coating Antibody to rat IL-2 (100 ug/ml)
- 1 vial (22 ul) Streptavidin-HRP
- 1 vial (55 ul) Biotin-Conjugate anti- rat IL-2 monoclonal antibody
- 1 vial rat IL-2 Standard protein lyophilized, 40 ng/ml upon reconstitution
- 2 vials (50 ml) Sample Diluent
Preparation and Storage
Shipping
The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below.
Stability & Storage
Storage of components varies. See protocol for specific instructions.
Background: IL-2
Long Name
Interleukin 2
Alternate Names
Aldesleukin, IL2, Proleukin, TCGF
Gene Symbol
IL2
Additional IL-2 Products
Product Documents for Rat IL-2 ELISA Kit (Colorimetric)
Product Specific Notices for Rat IL-2 ELISA Kit (Colorimetric)
This product is for research use only and is not approved for use in humans or in clinical diagnosis. ELISA Kits are guaranteed for 6 months from date of receipt.
Loading...
Loading...
Loading...
Loading...