Recombinant Human GFAP GST (N-Term) Protein
Novus Biologicals, part of Bio-Techne | Catalog # H00002670-Q01

Key Product Details
Source
Wheat germ
Tag
GST (N-Term)
Conjugate
Unconjugated
Applications
ELISA, Affinity Purification, Microarray, Western Blot
Product Specifications
Description
A recombinant protein with a N-terminal GST tag corresponding to the amino acid sequence 131-230 of Human GFAP
Source: Wheat Germ (in vitro)
Amino Acid Sequence: TANSARLEVERDNLAQDLATVRQKLQDETNLRLEAENNLAAYRQEADEATLARLDLERKIESLEEEIRFLRKIHEEEVRELQEQLARQQVHVELDVAKPD
Purity
>80% by SDS-PAGE and Coomassie blue staining
Predicted Molecular Mass
36.74 kDa.
Disclaimer note: The observed molecular weight of the protein may vary from the listed predicted molecular weight due to post translational modifications, post translation cleavages, relative charges, and other experimental factors.
Disclaimer note: The observed molecular weight of the protein may vary from the listed predicted molecular weight due to post translational modifications, post translation cleavages, relative charges, and other experimental factors.
Activity
This protein was produced in an in vitro wheat germ expression system that should preserve correct conformational folding that is necessary for biological function. While it is possible that this protein could display some level of activity, the functionality of this protein has not been explicitly measured or validated.
Protein / Peptide Type
Recombinant Protein
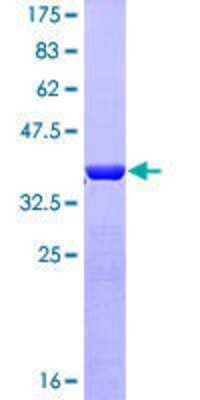
Scientific Data Images for Recombinant Human GFAP GST (N-Term) Protein
12.5% SDS-PAGE Stained with Coomassie Blue.
Formulation, Preparation and Storage
H00002670-Q01
| Preparation Method | in vitro wheat germ expression system |
| Formulation | 51 mM Tris-HCl (pH 8), 10 mM reduced Glutathione, in the elution buffer |
| Preservative | No Preservative |
| Concentration | Please see the vial label for concentration. If unlisted please contact technical services. |
| Shipping | The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Store at -80C. Avoid freeze-thaw cycles. |
Background: GFAP
An increase in GFAP levels is often associated with neuroinflammation which results in the activation and proliferation of astroglia cell population (1,2). GFAP expression is also observed in brains of patients with neurodegenerative diseases including Alzheimer's and Parkinson's, epilepsy disorders, and brain injuries (1-4). Lesion sites associated with neurodegeneration can exhibit an array of gliosis characteristics from glial scarring with reduced astrocyte proliferation to activated, GFAP-positive astrocytes surrounding amyloid plaques (2). Furthermore, the GFAP gene is a target of single nucleotide polymorphisms in the coding region, considered a gain-of-function mutation, characterized by astrocytic inclusions, termed Rosenthal fibers, resulting in Alexander Disease (1-4). GFAP is also a center of many post-translational modifications, such as phosphorylation, which can alter various aspects of filament assembly (1,4).
References
1. Yang, Z., & Wang, K. K. (2015). Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends in Neurosciences. https://doi.org/10.1016/j.tins.2015.04.003
2. Hol, E. M., & Capetanaki, Y. (2017). Type III Intermediate Filaments Desmin, Glial Fibrillary Acidic Protein (GFAP), Vimentin, and Peripherin. Cold Spring Harbor Perspectives in Biology. https://doi.org/10.1101/cshperspect.a021642
3. Potokar, M., Morita, M., Wiche, G., & Jorgacevski, J. (2020). The Diversity of Intermediate Filaments in Astrocytes. Cells. https://doi.org/10.3390/cells9071604
4. Viedma-Poyatos, a., Pajares, M. A., & Perez-Sala, D. (2020). Type III intermediate filaments as targets and effectors of electrophiles and oxidants. Redox Biology. https://doi.org/10.1016/j.redox.2020.101582
Long Name
Glial Fibrillary Acidic Protein
Alternate Names
ALXDRD, FLJ45472, GFAP, GFAP astrocytes, glial fibrillary acidic protein
Gene Symbol
GFAP
Additional GFAP Products
Product Documents for Recombinant Human GFAP GST (N-Term) Protein
Product Specific Notices for Recombinant Human GFAP GST (N-Term) Protein
This product is produced by and distributed for Abnova, a company based in Taiwan.
This product is for research use only and is not approved for use in humans or in clinical diagnosis. This product is guaranteed for 1 year from date of receipt.
Loading...
Loading...
Loading...
Loading...