Recombinant Escherichia coli Metalloprotease/StcE His, CF
R&D Systems, part of Bio-Techne | Catalog # 11406-MP
His-tag

Key Product Details
Product Specifications
Source
E. coli-derived e. coli StcE protein
Ala36-Lys898 with a N-terminal Met and 6-His tag
Ala36-Lys898 with a N-terminal Met and 6-His tag
Purity
>85%, by SDS-PAGE visualized with Silver Staining and quantitative densitometry by Coomassie® Blue Staining.
Endotoxin Level
<0.10 EU per 1 μg of the protein by the LAL method.
N-terminal Sequence Analysis
Met & Arg415
Predicted Molecular Mass
97 kDa & 54 kDa
SDS-PAGE
86-95 & 53-58 kDa, under reducing conditions.
Activity
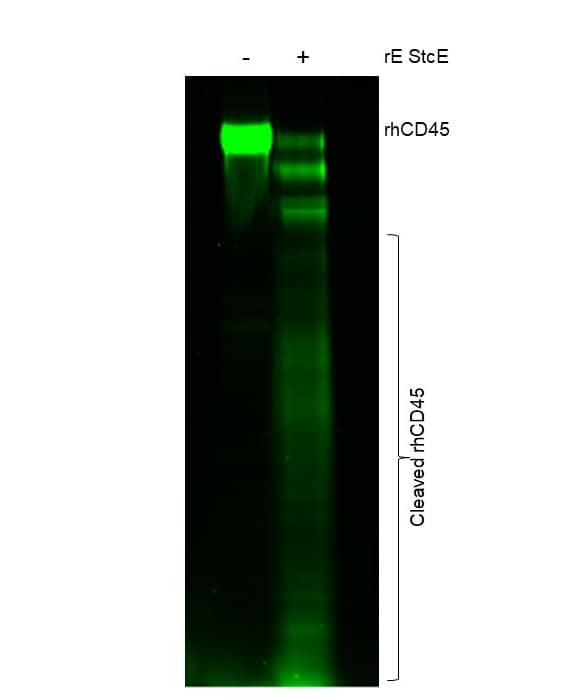
Measured by its ability to cleave Recombinant CD45 Protein at specific O-glycan sites.
One μg of Recombinant E. coli StcE will cleave >60% of Recombinant CD45 Protein with labeled O-glycan
(Catalog #
1430-CD), as measured under the described conditions.
Scientific Data Images for Recombinant Escherichia coli Metalloprotease/StcE His, CF
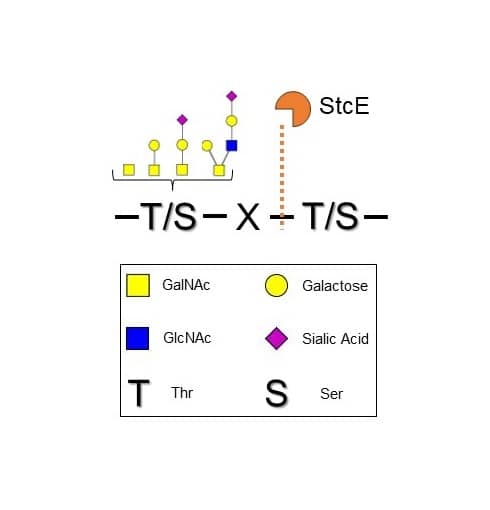
Recombinant E. coli StcE His-tag Enzyme Activity Diagram.
Recombinant E. coli StcE His-tag cleaves glycoproteins C-terminally to glycosylated Ser/Thr residues that are near the cleavage residue. Specificity for O-glycan complexity is broad. For simplicity not all O-glycans may be represented.Recombinant E. coli StcE His-tag Enzyme Activity Assay.
Recombinant Human CD45 Protein, CF (1430-CD) was first cleaved using Recombinant E. coli StcE (Cat # 11406-MP). Following cleavage, Recombinant C. perfringens Neuraminidase Protein, CF (5080-NM) was used to remove intact Sialic Acid. O-glycans were then labeled using Recombinant Human ST3GAL2 Protein, CF (7275-GT) and CMP-Cy3-Sialic Acid (ES402). Samples were then run on a SDS-PAGE gel and imaged using the green fluorescent channel.Formulation, Preparation and Storage
11406-MP
| Formulation | Supplied as a 0.2 μm filtered solution in Tris and NaCl. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: StcE
References
- Yu, A. et al. (2012). Structure. 20:707.
- Grys, TE. et al. (2005). Infection and Immunity. 73:1295.
- Furniss, RCD. et al. (2018). J Biol. Chem. 293:17188.
- Hews, CL et al. (2017). Cell. Microbiol. 19:e12717.
- Malaker, SA. et al. (2019). Proc. Natl. Acad. Sci. USA. 116:7278.
- Taleb, V. et al. (2022). Nat. Commun. 13:4324.
- Shon, DJ. et al. (2020). Proc. Natl. Acad. Sci. USA. 117:21299.
- Pluvinage, B. et al. (2021). Proc. Natl. Acad. Sci. USA. 118:e2019220118.
Long Name
Metalloprotease stcE
UniProt
Additional StcE Products
Product Documents for Recombinant Escherichia coli Metalloprotease/StcE His, CF
Product Specific Notices for Recombinant Escherichia coli Metalloprotease/StcE His, CF
For research use only
Loading...
Loading...
Loading...