Recombinant Human ALDH1A2 His-tag, CF
R&D Systems, part of Bio-Techne | Catalog # 10132-DH

Key Product Details
Product Specifications
Source
Thr2-Ser518
with an N-terminal Met and 6-His tag
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
SDS-PAGE
Activity
The specific activity is >150 pmol/min/μg, as measured under the described conditions.
Scientific Data Images for Recombinant Human ALDH1A2 His-tag, CF
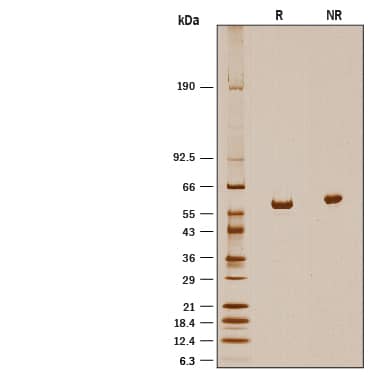
Recombinant Human ALDH1A2 His-tag SDS-PAGE
1 μg/lane of Recombinant Human ALDH1A2 His-tag was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by silver staining, showing a band at 58 kDa under reducing conditions.Formulation, Preparation and Storage
10132-DH
| Formulation | Supplied as a 0.2 μm filtered solution in Tris, NaCl, Glycerol and DTT. |
| Shipping | The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: ALDH1A2
Aldehyde dehydrogenases (ALDHs) are NAD(P)+-dependent enzymes that detoxify aldehydes by oxidizing them to carboxylic acids. Nineteen ALDHs are present in humans, expressed in a variety of organelles and having different substrate preferences (1). Human ALDH1A2 is a NAD-dependent, cytosolic member of the ALDH1A subfamily class 1 that forms active tetramers that synthesize retinoic acid from retinal. Each monomer has three functional regions: a catalytic domain containing the active site cysteine, an NAD-binding domain, and the oligomerization domain (2, 3). ALDH1A2 exhibits the highest substrate specificity and catalytic efficiency for retinal oxidation to retinoic acid (RA) (4) and thus plays an important role in regulation of RA production and signaling. ALDH1A2 null mice are embryonic lethal (5) and mutations in ALDH1A2 are associated with pathological conditions such as osteoarthris (6-8). Additionally, ALDH1A2 is suggested to play a role in several cancers. ALDH1A2 was a suggested tumor marker (9, 10) and predictor of prostate cancer relapse (11). Abnormally low levels of ALDH1A2 has been observed in several cancers including breast (12), squamous cell carcinoma of the head and neck (13), and specifically in high grade ovarian cancer, suggesting high expression may be associated with favorable prognosis (14). Overexpression of ALDH1A2 in cancer lines resulted in decreased proliferation and migration (14) supporting that ALDH1A2 plays a tumor suppressor role. In contrast, ALDH1A2 is reported to exhibit high expression correlated with worse overall survival in non-small-cell lung cancer (15) and chemoresistant cancer stem cells in neuroblastoma (16, 17). Pharmacologically specific inhibitors and activators of ALDH1A2 are of interest due to its implied role in a variety of diseases and proliferation and drug resistance (1, 3, 15, 18).
References
- Koppaka, V. et al. (2012) Pharmacol. Rev. 64:520.
- Perez-Miller, S. J. and T. D. Hurley (2003) Biochemistry. 42:7100.
- Chen, Y. et al. (2018) ACS Chem. Biol. 13:582.
- Duester, G. (2001) Chem. Biol. Interact. 130:469.
- Niedereither, K. et al. (1999) Nat. Genet. 21:444.
- Pavan, M. et al. (2009) B.M.C. Med. Genet. 10:113.
- Styrkarsdottir, U. et al. (2014) Nat. Genet. 46:498.
- Shepherd, C. et. al. (2018) Arthritis Rheumatol. 70:1577.
- Kim, H. et al. (2005) Cancer Res. 65:8118.
- Touma, S. E. et al. (2009) Biochem. Pharmacol. 78:1127.
- Nim, H. T. et al. (2017) Front. Oncol. 7:30.
- Mira, Y. L. R. et al. (2000) J. Cell Physiol. 185:302.
- Seidensaal, K. et al. (2015) Mol. Cancer 14:204.
- Wang, Y. et al. (2018) Onco. Targets Ther. 31:599.
- You, Q. et al. (2015) Drug Des. Devel. Ther. 9:5087.
- Hartomo, T. B. et al. (2015) Int. J. Oncol. 46:1089.
- Singh, S. et al. (2015) Adv. Exp. Med. Biol. 815:281.
- Moreb, J. S. et al. (2012) Chem. Biol. Interact. 195:52.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional ALDH1A2 Products
Product Documents for Recombinant Human ALDH1A2 His-tag, CF
Product Specific Notices for Recombinant Human ALDH1A2 His-tag, CF
For research use only