Recombinant Human C1qTNF10/C1qL2 Protein, CF
R&D Systems, part of Bio-Techne | Catalog # 9189-TN

Key Product Details
Source
E. coli
Accession #
Structure / Form
Noncovalently-linked homotrimer
Conjugate
Unconjugated
Applications
Bioactivity
Product Specifications
Source
E. coli-derived human C1qTNF10/C1qL2 protein
Phe154-Asp287, with an N-terminal Met and 6-His tag
Phe154-Asp287, with an N-terminal Met and 6-His tag
Purity
>70%, by SDS-PAGE visualized with Silver Staining and quantitative densitometry by Coomassie® Blue Staining.
Endotoxin Level
<0.10 EU per 1 μg of the protein by the LAL method.
N-terminal Sequence Analysis
Met
Predicted Molecular Mass
16 kDa
SDS-PAGE
14 kDa, reducing conditions
Activity
Measured by its binding ability in a functional ELISA.
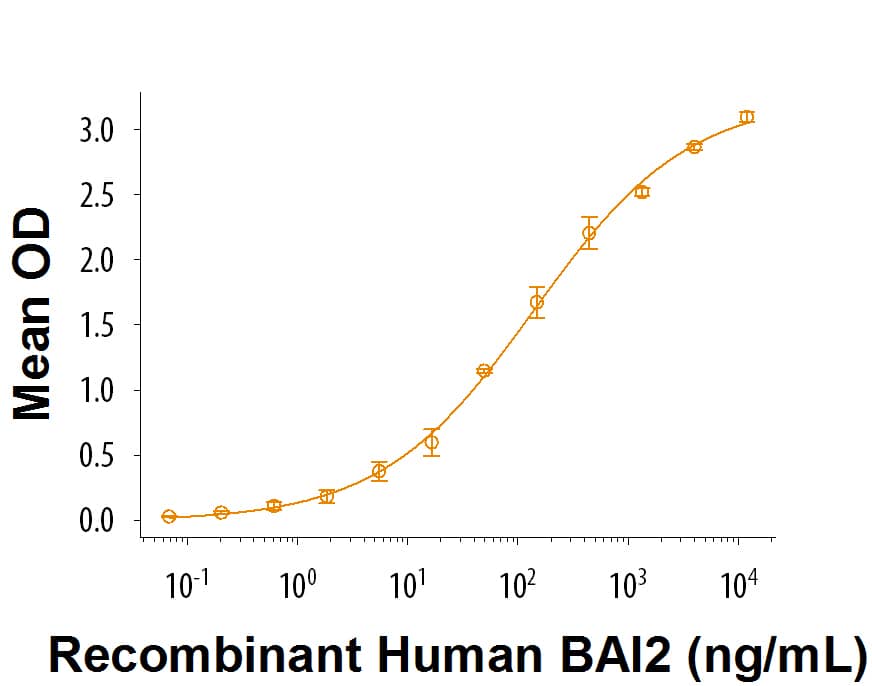
When Recombinant Human C1qTNF10/C1qL2 is immobilized at 2 μg/mL, 100 μL/well, the concentration of Recombinant Human BAI2 Protein Fc Chimera (Catalog # 9338-BA) that produces 50% of the optimal binding response is approximately 0.075-0.45 μg/mL.
When Recombinant Human C1qTNF10/C1qL2 is immobilized at 2 μg/mL, 100 μL/well, the concentration of Recombinant Human BAI2 Protein Fc Chimera (Catalog # 9338-BA) that produces 50% of the optimal binding response is approximately 0.075-0.45 μg/mL.
Scientific Data Images for Recombinant Human C1qTNF10/C1qL2 Protein, CF
Recombinant Human C1qTNF10/C1qL2 Protein Bioactivity
When Recombinant Human C1qTNF10 (Catalog # 9189-TN) is coated at 2 μg/mL, 100 μL/well, Recombinant Human BAI2 Fc Chimera binds with a ED50 of 0.075-0.45 μg/mL.Formulation, Preparation and Storage
9189-TN
| Formulation | Lyophilized from a 0.2 μm filtered solution in Tris, NaCl, TCEP and Trehalose. |
| Reconstitution |
Reconstitute at 500 μg/mL in water.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: C1qTNF10/C1qL2
References
- Grebrehiwet, B. et al. (2012) Front. Immunol. 5:3.
- Yuzaki, M. (2001) Eur. J. Neurosci. 32:191.
- Peterson, J.M. et al. (2009) Biochem. Biophys. Res. Commun. 388:360.
- Wei, Z. et al. (2011) J. Biol. Chem. 286:15652.
- Wei, Z. et al. (2013) J. Biol. Chem. 288:10214.
- Wong, G.W. et al. (2008) Biochem. J. 416:161.
- Bolliger, M.F. et al. (2011) Proc. Natl. Acad. Sci. USA 108:2534.
- Matsuda, K. et al. (2016) Neuron 90:752.
Long Name
C1q And Tumor Necrosis Factor Related Protein 10
Alternate Names
C1qL2
Gene Symbol
C1QL2
UniProt
Additional C1qTNF10/C1qL2 Products
Product Documents for Recombinant Human C1qTNF10/C1qL2 Protein, CF
Product Specific Notices for Recombinant Human C1qTNF10/C1qL2 Protein, CF
For research use only
Loading...
Loading...
Loading...