Recombinant Human CD40 Ligand/TNFSF5 Avi-tag His-tag, CF
R&D Systems, part of Bio-Techne | Catalog # AVI11596

Key Product Details
Source
Accession #
Structure / Form
Conjugate
Applications
Product Specifications
N-terminal Sequence Analysis
Predicted Molecular Mass
SDS-PAGE
Scientific Data Images for Recombinant Human CD40 Ligand/TNFSF5 Avi-tag His-tag, CF
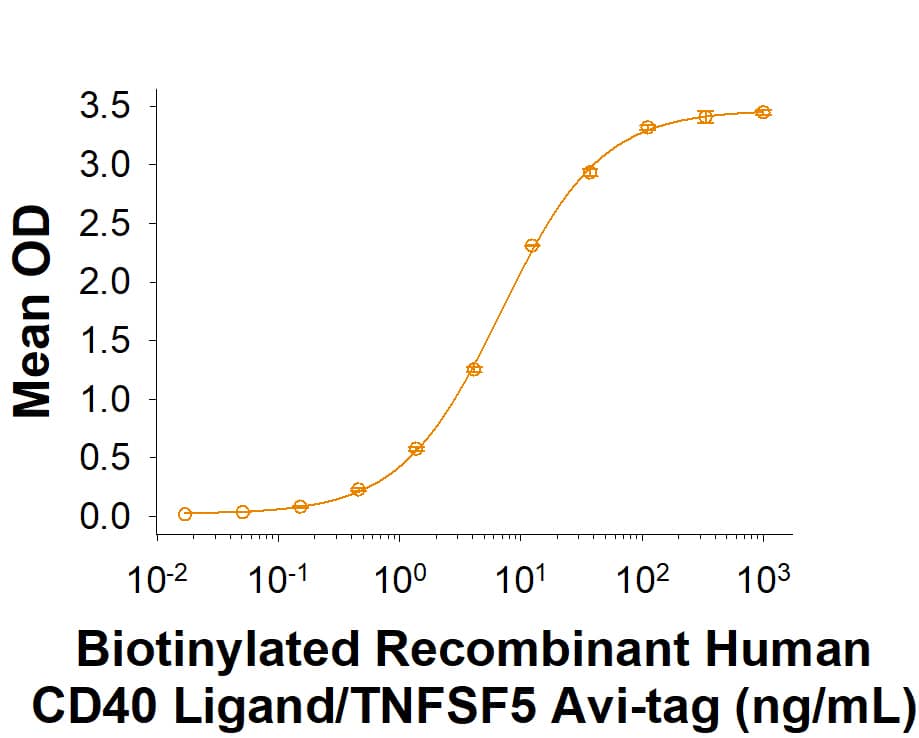
Biotinylated Recombinant Human CD40 Ligand/TNFSF5 Avi-tag His-tag Protein Binding Activity.
Biotinylated Recombinant Human CD40 Ligand/TNFSF5 Avi-tag His-tag Protein (Catalog # AVI11596) binds Recombinant Human CD40/TNFRSF5 Fc Chimera (1493-CDB) with an ED50 of 2.50-30.0 ng/mL.Biotinylated Recombinant Human CD40 Ligand/TNFSF5 Avi-tag His-tag Protein SDS-PAGE.
2 μg/lane of Biotinylated Recombinant Human CD40 Ligand/TNFSF5 Avi-tag His-tag Protein (Catalog # AVI11596) was resolved with SDS-PAGE under reducing (R) condition and visualized by Coomassie® Blue staining, showing bands at 18-25 kDa.Formulation, Preparation and Storage
AVI11596
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS and EDTA with Trehalose. |
| Reconstitution | Reconstitute at 250 μg/mL in sterile water. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Store the unopened product at -20 to -70 °C. Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Do not use past expiration date. |
Background: CD40 Ligand/TNFSF5
CD40 Ligand, also known as TNFSF, CD154, TRAP, and gp39, is a 34-39 kDa type II transmembrane glycoprotein that belongs to the TNF superfamily (1-3). Mature human CD40 Ligand consists of a 22 amino acid (aa) cytoplasmic domain, a transmembrane segment, and an 215 aa extracellular region (4, 5). The extracellular domain of human CD40 Ligand shares 74% and 76% aa sequence identity with mouse and rat CD40 Ligand, respectively. Similar to other TNF superfamily members, CD40 Ligand forms a bioactive homotrimer, both as membrane bound and soluble forms (6-9). The 18 kDa soluble form (aa 113-261) arises from proteolytic processing. Mutation and alternative splicing generate additional forms of CD40 Ligand that are often truncated or non-trimerizable (8). CD40 Ligand is expressed on platelets, as well as on activated T cells and B cells, basophils, eosinophils, fibroblasts, mast cells, monocytes, natural killer cells, vascular endothelial cells, and smooth muscle cells. CD40 Ligand binds to CD40, which is expressed on the surface of B cells, dendritic cells, macrophages, monocytes, platelets, endothelial, and epithelial cells (10). The interaction of CD40 Ligand with CD40 initiates signaling in both CD40 and CD40 Ligand expressing cells (11). CD40 ligation by CD40 Ligand promotes B cell activation and T cell-dependent humoral responses (12, 13). CD40 Ligand dysregulation on T cells and antigen presenting cells contributes to the immune deficiency associated with HIV infection and AIDS (14, 15). It is also implicated in the pathology of multiple cardiovascular diseases including atherosclerosis, atherothrombosis, and restenosis (16, 17). Our Avi-tag Biotinylated human CD40 Ligand features biotinylation at a single site contained within the Avi-tag, a unique 15 amino acid peptide. Protein orientation will be uniform when bound to streptavidin-coated surface due to the precise control of biotinylation and the rest of the protein is unchanged so there is no interference in the protein's bioactivity.
References
- Zhang, G. (2004) Curr. Opin. Struct. Biol. 14:154.
- Hehlgans, T. and K. Pfeffer (2005) Immunology 115:1.
- Quezada, S.A. et al. (2004) Annu. Rev. Immunol. 22:307.
- Graf, D. et al. (1992) Eur. J. Immunol. 22:3191.
- Hollenbaugh, D. et al. (1992) EMBO J. 11:4313.
- Khandekar, S.S. et al. (2001) Protein Expr. Purif. 23:301.
- Pietravalle, F. et al. (1996) J. Biol. Chem. 271:5965.
- Garber, E. et al. (1999) J. Biol. Chem. 274:33545.
- Vakkalanka, R.K. et al. (1999) Arthritis Rheum. 42:871.
- van Kooten, C. and J. Banchereau (1997) Curr. Opin. Immunol. 9:330.
- Eissner, G. et al. (2004) Cytokine Growth Factor. Rev. 15:353.
- Rickert, R.C. et al. (2011) Immunol. Rev. 244:115.
- Elgueta, R. et al. (2009) Immunol. Rev. 229:152.
- Kornbluth, R.S. (2000) J. Leukoc. Biol. 68:373.
- Chougnet, C. (2003) J. Leukoc. Biol. 74:702.
- Pamukcu, B. et al. (2011) Ann. Med. 43:331.
- Hassan, G.S. et al. (2012) Immunobiology 217:521.
Alternate Names
Gene Symbol
UniProt
Additional CD40 Ligand/TNFSF5 Products
- All Products for CD40 Ligand/TNFSF5
- CD40 Ligand/TNFSF5 cDNA Clones
- CD40 Ligand/TNFSF5 ELISA Kits
- CD40 Ligand/TNFSF5 Luminex Assays
- CD40 Ligand/TNFSF5 Lysates
- CD40 Ligand/TNFSF5 Primary Antibodies
- CD40 Ligand/TNFSF5 Proteins and Enzymes
- CD40 Ligand/TNFSF5 Simple Plex
- CD40 Ligand/TNFSF5 Small Molecules and Peptides
Product Documents for Recombinant Human CD40 Ligand/TNFSF5 Avi-tag His-tag, CF
Product Specific Notices for Recombinant Human CD40 Ligand/TNFSF5 Avi-tag His-tag, CF
For research use only