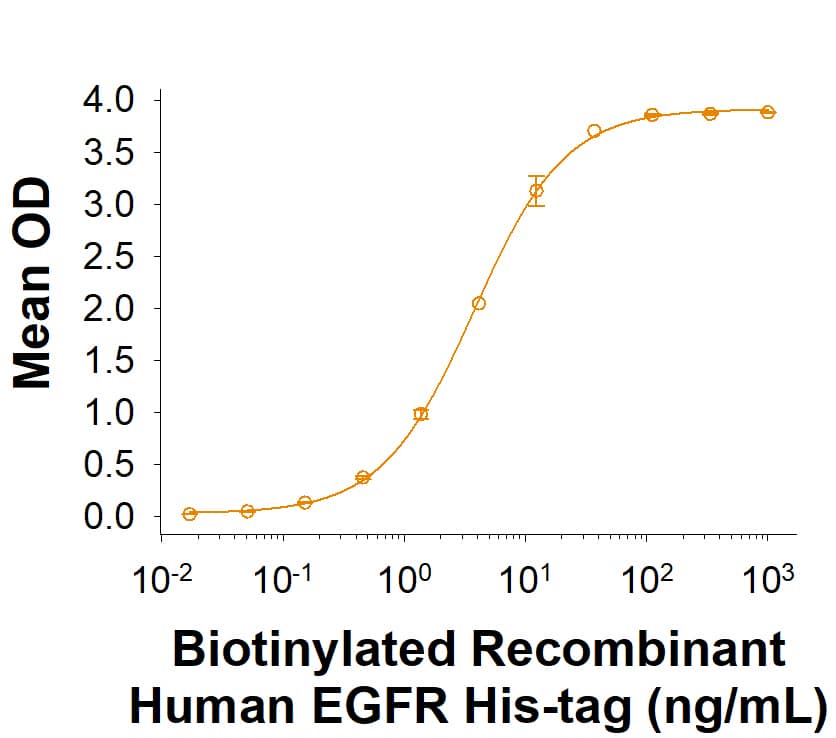
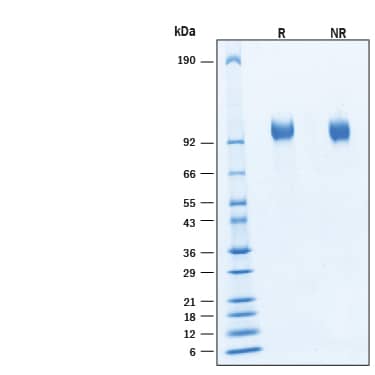
Epidermal growth factor receptor (EGFR), also known as HER-1 and ErbB1, is a member of a subfamily of receptor tyrosine kinases comprised of four members: EGFR, ErbB2 (Neu, HER-2), ErbB3 (HER-3), and ErbB4 (HER-4). All family members are type I transmembrane glycoproteins with an extracellular domain (ECD) containing two cysteine-rich domains separated by a spacer region and a cytoplasmic domain containing a tyrosine kinase domain followed by multiple tyrosine autophosphorylation sites (1, 2). Several soluble isoforms lacking the intracellular domain are generated by alternate splicing, along with a tumor specific mutant EGFRvIII, are known to exist (3-5). The ECD of mature, full-length EGFR shares 88% and 89% amino acid sequence identity with mouse and rat EGFR, respectively. EGFR binds a subset of the EGF family ligands, including EGF, amphiregulin, TGF-alpha, betacellulin, epiregulin, HB-EGF, and epigen (1, 2). Ligand binding induces EGFR homodimerization as well as heterodimerization with ErbB2, resulting in kinase activation, heterodimerization tyrosine phosphorylation and cell signaling (6-8). EGFR can also be recruited to form heterodimers with the ligand‑activated ErbB3 or ErbB4. EGFR signaling regulates multiple biological functions including cell proliferation, differentiation, motility, and apoptosis (6-8). EGFR is overexpressed in a wide variety of tumors, with EGFRvIII overexpressed particularly in glioblastoma multiforme (GMB) and is the target of several anti-cancer therapeutics (5,9,10).