Recombinant Human LIF, Animal-Free Protein
R&D Systems, part of Bio-Techne | Catalog # Qk036

Key Product Details
Product Specifications
Source
Purity
Endotoxin Level
Predicted Molecular Mass
SDS-PAGE
Activity
Scientific Data Images for Recombinant Human LIF, Animal-Free Protein
Recombinant Human LIF, Animal-Free Protein Bioactivity
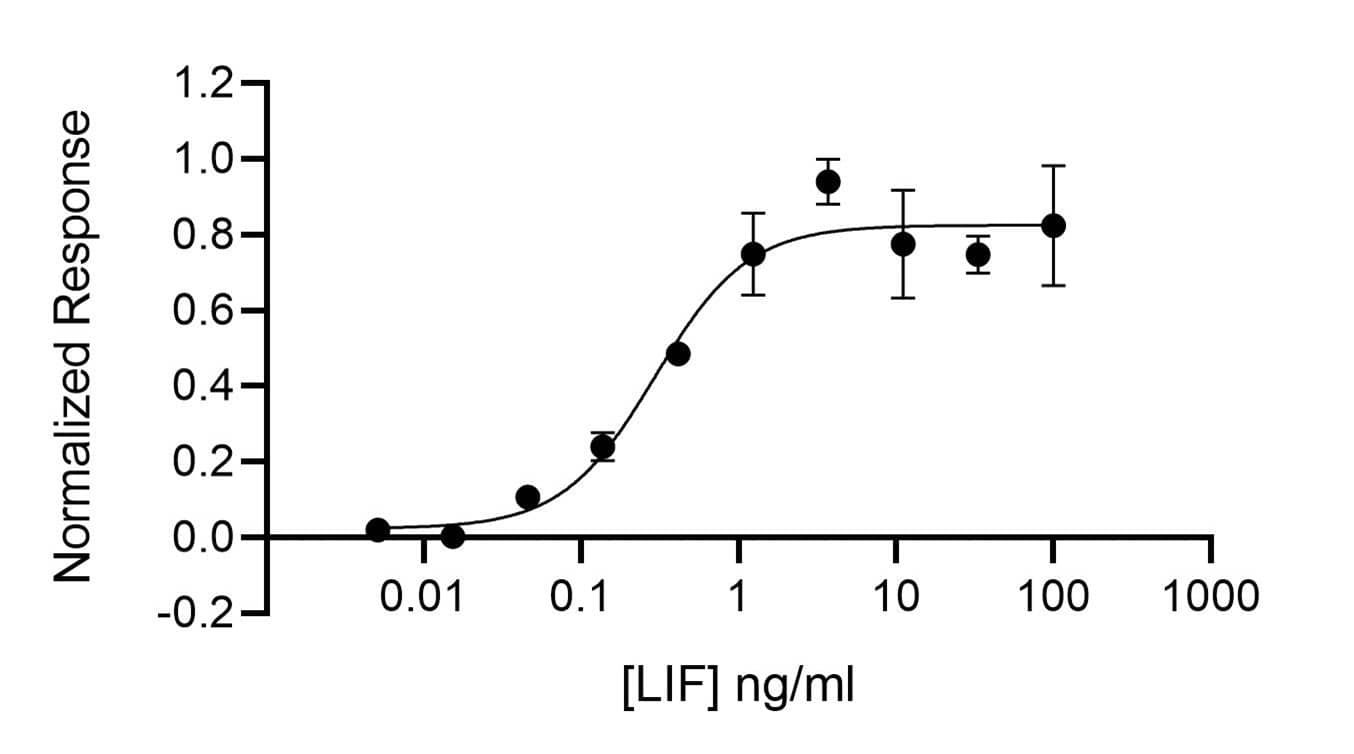
Human LIF activity is determined using the LIF-responsive firefly luciferase reporter assay (*). HEK293T cells are treated in triplicate with a serial dilution of LIF overnight. Firefly luciferase activity is measured and normalized to the control Renilla luciferase activity. EC50 = 0.29 ng/ml (17.3 pM).*Promega pGL4.33[luc2P/SRE/Hygro] #E1340Recombinant Human LIF, Animal-Free Protein SDS-PAGE
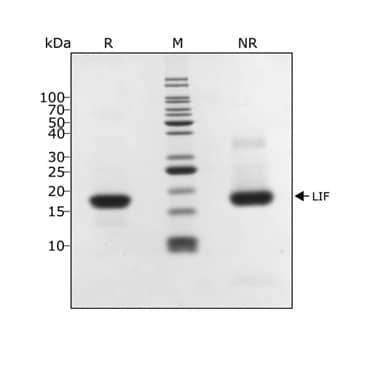
Human LIF protein (Qk036) migrates as a single band at 20 kDa in non-reducing (NR) and upon reduction (R). Purified recombinant human LIF protein (3 μg) was resolved using 15% w/v SDS-PAGE in reduced (+ beta-mercaptoethanol, R) and non-reduced conditions (NR) and stained with Coomassie Brilliant Blue R250.Formulation, Preparation and Storage
Qk036
| Formulation | Lyophilized from acetonitrile/TFA |
| Reconstitution | Resuspend in 10mM HCl at >100 µg/ml, prepare single use aliquots, add carrier protein if desired. |
| Shipping | The product is shipped lyophilized at ambient temperture, on ice blocks or dry ice. Shipping at ambient temperture does not affect the bioactivity or stability of the protein. Upon reciept, store immediately at the conditions stated below. |
| Stability & Storage | Store lyophilized protein between -20 and -80 °C until the date of expiry. Avoid freeze-thaw cycles. |
Background: LIF
LIF (leukemia inhibitory factor) is a widely expressed, highly and variably glycosylated, 32‑62 kDa, monomeric, pleiotropic cytokine in the IL‑6 family of helical cytokines (1‑4). The first exon encoding the signal sequence is alternately spliced, resulting in LIF-D, LIF-M, and LIF‑T mRNAs that produce secreted, extracellular matrix‑associated, and intracellular forms, respectively (5). LIF-D and LIF-M mRNAs produce identical 180 amino acid (aa) mature sequences (5). Mature human LIF (180 aa) shares 78%, 82%, 91%, 88 and 87% aa sequence identity with mouse, rat, canine, bovine, and porcine LIF, respectively. The LIF receptor is a heterodimer of a type I transmembrane ligand‑binding subunit, LIFR (gp190), and the type I transmembrane signal transducing subunit, gp130, signaling especially through STAT3 and JAK kinases (3, 4, 6). Gp130 and members of the LIFR family also mediate the biological effects of Oncostatin M, Cardiotrophin‑1, Galectin‑10, CNTF, IL‑6,
IL‑11, and IL‑27 (3, 6). A soluble LIFR has been reported in the mouse (7). Depending on the cells and their context, LIF either opposes or favors differentiation (3, 8). LIF produced by the uterine endometrium supports successful implantation of the embryo, promotes proliferation and maintenance of pluripotency in embryonic stem cells, and favors proliferation of progenitor cell types such as hematopoietic stem cells (3, 6, 8). However, excess LIF blocks differentiation of embryoid bodies, indicating the importance of LIF regulation (3, 6). LIF is produced by CD4+ T cells in response to activation, and is required by the thymic epithelium to support T cell maturation (3, 4). LIF expression is up‑regulated by neuronal injury, and promotes motor neuron survival and oligodendrocyte myelination (3, 4, 9). LIF is produced by the adrenal cortex and likely enhances its production of cortisol and aldosterone (10). LIF can function as an autocrine growth factor in some pancreatic cancers, but induces differentiation in the myeloid leukemic cell line M1 (2, 11). Tumor LIF can also induce formation of immunosuppressive tumor‑associated macrophages (12). LIF promotes endometrial remodeling and differentiation of adipocytes and cardiac smooth muscle cells (3, 4). It promotes regulatory T cell and inhibits Th17 cell differentiation, thus promoting tolerance, down‑regulating inflammation, and contributing to immune tolerance during pregnancy and in the nervous system (3, 4, 6, 8).
References
- Gough, N.M. et al. (1988) Proc. Natl. Acad. Sci. USA 85:2623.
- Moreau, J.F. et al. (1988) Nature 336:690.
- Trouillas, M. et al. (2009) Eur. Cytokine Netw. 20:51.
- Metcalfe, S.M. (2011) Genes Immun. 12:157.
- Voyle, R.B. et al. (1999) Exp. Cell Res. 249:199.
- Cheng, J.G. et al. (2001) Proc. Natl. Acad. Sci. USA 98:8680.
- Tomida, M. et al. (1993) FEBS lett. 334:193.
- Paiva, P. et al. (2009) Cytokine Growth Factor Rev. 20:319.
- Slaets, H. et al. (2010) Trends Mol. Med. 16:493.
- Bamberger, A.M. et al. (2000) Mol. Cell. Endocrinol. 162:145.
- Kamohara, H. et al. (2007) Int. J. Oncol. 30:977.
- Duluc, D. et al. (2007) Blood 110:4319.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional LIF Products
Product Documents for Recombinant Human LIF, Animal-Free Protein
Product Specific Notices for Recombinant Human LIF, Animal-Free Protein
The above product was manufactured, tested and released by R&D System's contract manufacturer, Qkine Ltd, at 1 Murdoch House, Cambridge, UK, CB5 8HW. The product is for research use only and not for the diagnostic or theraputic use.
For research use only