Recombinant Human Noggin, Animal-Free Protein
R&D Systems, part of Bio-Techne | Catalog # Qk034

Key Product Details
Product Specifications
Source
Purity
Endotoxin Level
Predicted Molecular Mass
SDS-PAGE
Activity
Mycoplasma
Scientific Data Images for Recombinant Human Noggin, Animal-Free Protein
Recombinant Human Noggin, Animal-Free Protein Bioactivity
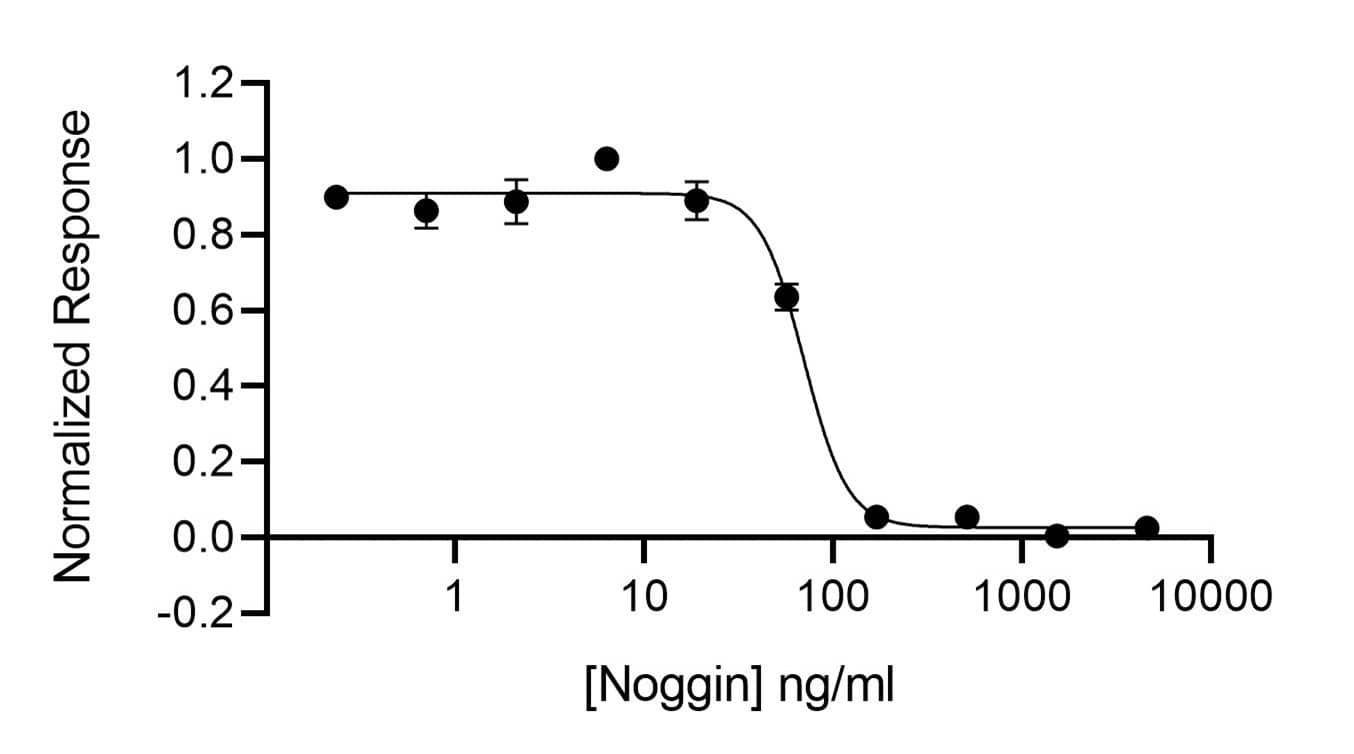
Human Noggin is a BMP inhibitor, and its activity is determined by inhibition of BMP2 (Qk007) activity in a BMP-2 responsive firefly luciferase reporter assay. HEK293T cells are treated in triplicate with a serial dilution of Noggin and a standard concentration of BMP2 for 6 hours. Firefly luciferase activity is measured and normalized to the control Renilla luciferase activity.EC50 = 70.1 ng/ml (1.5 nM).Recombinant Human Noggin, Animal-Free Protein SDS-PAGE
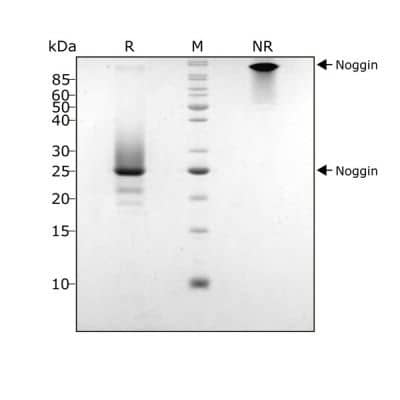
Noggin protein (Qk034) has an unusual migration in non-reduced (NR) SDS-PAGE due to the non-covalent dimer which is the active protein. Similar migration in SDS-PAGE is seen for Gremlin-1, a related BMP antagonist. The identity of the purified dimeric protein was confirmed using mass spectrometry. Upon reduction, the protein monomer migrates at 23 kDaPurified recombinant human Noggin protein (7 μg) was resolved using 15% w/v SDS-PAGE in reduced (+ beta-mercaptoethanol, R) and non-reduced conditions (NR) and stained with Coomassie Brilliant Blue R250.Formulation, Preparation and Storage
Qk034
| Formulation | Lyophilized from acetonitrile/TFA |
| Reconstitution | Resuspend in 10mM HCl at >100 µg/ml, prepare single use aliquots, add carrier protein if desired. |
| Shipping | The product is shipped lyophilized at ambient temperture, on ice blocks or dry ice. Shipping at ambient temperture does not affect the bioactivity or stability of the protein. Upon reciept, store immediately at the conditions stated below. |
| Stability & Storage | Store lyophilized protein between -20 and -80 °C until the date of expiry. Avoid freeze-thaw cycles. |
Background: Noggin
Noggin is a secreted homodimeric glycoprotein that is an antagonist of bone morphogenetic proteins (BMPs) (1, 2). Human Noggin cDNA encodes a 232 amino acid (aa) precursor protein; cleavage of a 19 aa signal peptide generates the 213 aa mature protein which contains an N-terminal acidic region, a central basic heparin-binding segment and a C-terminal cysteine-knot structure (2). Secreted Noggin probably remains close to the cell surface due to its binding of heparin-containing proteoglycans (3). Noggin is very highly conserved among vertebrates, such that mature human Noggin shares 99%, 99%, 98%, 97% and 89% aa sequence identity with mouse, rat, bovine, equine and chicken Noggin, respectively. Noggin binds some BMPs such as BMP-4 with high affinity and others such as BMP-7 with lower affinity. It antagonizes BMP bioactivities by blocking epitopes on BMPs that are needed for binding to both type I and type II receptors (2, 4). During embryogenesis, Noggin antagonizes specific BMPs at defined times, for example, during neural tube, somite and cardiomyocyte growth and patterning (5-7). During skeletal development, Noggin prevents chondrocyte hyperplasia, thus allowing proper formation of joints (4). Mutations within the cysteine-knot region of human Noggin are linked to multiple types of skeletal dysplasias that result in apical joint fusions (8). Noggin is expressed in defined areas of the adult central nervous system and peripheral tissues such as lung, skeletal muscle and skin (1). During culture of human embryonic stem cells (hESC) or neural stem cells under certain conditions, addition of Noggin to antagonize BMP activity may allow stem cells to proliferate while maintaining their undifferentiated state, or alternatively, to differentiate into dopaminergic neurons (6, 9 - 13). Noggin also appears to maintain adult stem cell populations in-vivo, for example, maintaining neural stem cells within the hippocampus (13).
References
- Valenzuela, D.M. et al. (1995) J. Neurosci. 15:6077.
- Groppe, J. et al. (2002) Nature 420:636.
- Paine-Saunders, S et al. (2002) J. Biol. Chem. 277:2089.
- Brunet, L. J. et al. (1998) Science 280:1455.
- McMahon, J. A. et al. (1998) Genes Dev. 12:1438.
- Itsykson, P. et al. (2005) Mol. Cell. Neurosci. 30:24.
- Yuasa, S. et al. (2005) Nat. Biotechnol. 23:607.
- Gong, Y. et al. (1999) Nat. Genet. 21:302.
- Xu, R.-H. et al. (2005) Nat. Methods 2:185.
- Wang, G. et al. (2005) Biochem. Biophys. Res. Commun. 330:934.
- Chaturvedi, G. et al. (2009) Cell Prolif. 42:425.
- Chiba, S. et al. (2008) Stem Cells 26:2810.
- Bonaguidi, M.A. et al. (2008) J. Neurosci. 28:9194.
Alternate Names
Gene Symbol
UniProt
Additional Noggin Products
Product Documents for Recombinant Human Noggin, Animal-Free Protein
Product Specific Notices for Recombinant Human Noggin, Animal-Free Protein
The above product was manufactured, tested and released by R&D System's contract manufacturer, Qkine Ltd, at 1 Murdoch House, Cambridge, UK, CB5 8HW. The product is for research use only and not for the diagnostic or theraputic use.
For research use only