Recombinant Human Siglec-10 Fc Chimera Avi-tag Protein, CF
R&D Systems, part of Bio-Techne | Catalog # AVI2130
Biotinylated

Key Product Details
Learn more about Avi-tag Biotinylated Proteins
Source
CHO
Accession #
Structure / Form
Disulfide-linked homodimer, biotinylated via Avi-tag
Conjugate
Biotin
Applications
Bioactivity
Product Specifications
Source
Chinese Hamster Ovary cell line, CHO-derived human Siglec-10 protein
| Human Siglec-10 (Met17-Thr546) Accession # Q96LC7.3 |
IEGRMD | Human IgG1 (Pro100-Lys330) |
Avi-tag |
| N-terminus | C-terminus | ||
Purity
>95%, by SDS-PAGE visualized with Silver Staining and quantitative densitometry by Coomassie® Blue Staining.
Endotoxin Level
<0.10 EU per 1 μg of the protein by the LAL method.
N-terminal Sequence Analysis
Met17
Predicted Molecular Mass
87 kDa
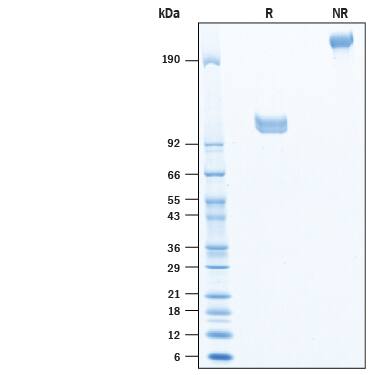
SDS-PAGE
95-118 kDa, under reducing conditions
Activity
The biotin to protein ratio is greater than 0.7 as determined by the HABA assay.
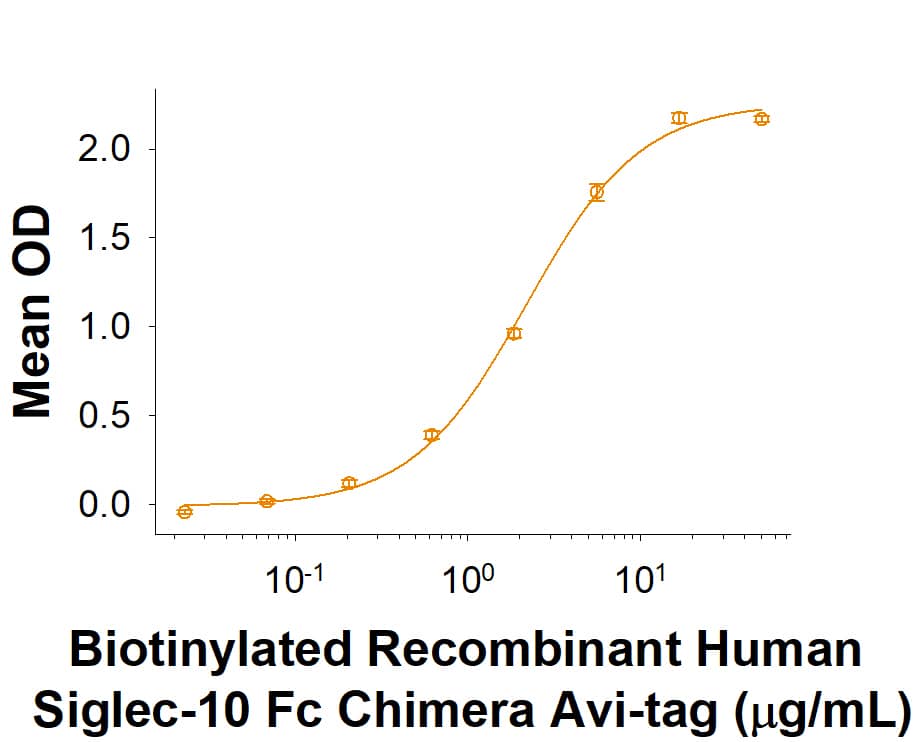
Measured by its binding ability in a functional ELISA.
When Recombinant Human CD52 Fc Chimera Protein (Catalog # 9116-CD) is immobilized at 5 μg/mL (100 µL/well), the concentration of Biotinylated Recombinant Human Siglec‑10 Fc Chimera Avi-tag (Catalog # AVI2130) that produces 50% of the optimal binding response is found to be approximately 0.75-4.50 μg/mL.
Measured by its binding ability in a functional ELISA.
When Recombinant Human CD52 Fc Chimera Protein (Catalog # 9116-CD) is immobilized at 5 μg/mL (100 µL/well), the concentration of Biotinylated Recombinant Human Siglec‑10 Fc Chimera Avi-tag (Catalog # AVI2130) that produces 50% of the optimal binding response is found to be approximately 0.75-4.50 μg/mL.
Scientific Data Images for Recombinant Human Siglec-10 Fc Chimera Avi-tag Protein, CF
Biotinylated Recombinant Human Siglec-10 Fc Chimera Avi-tag Protein Binding Activity.
When Recombinant Human CD52 Fc Chimera Protein (9116-CD) is immobilized at 5 μg/mL (100 µL/well), the concentration of Biotinylated Recombinant Human Siglec‑10 Fc Chimera Avi-tag (Catalog # AVI2130) that produces 50% of the optimal binding response is found to be approximately 0.75-4.50 μg/mL.Recombinant Human Siglec-10 Fc Chimera Avi-tag Protein SDS-PAGE
2 μg/lane of Recombinant Siglec-10 Fc Chimera Avi-tag (Catalog # AVI2130) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 95-118 kDa and 190-236 kDa, respectively.Formulation, Preparation and Storage
AVI2130
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 250 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: Siglec-10
References
- Varki, A. and Angata, T. (2006) Glycobiology, 16:1R.
- Li, N. et al. (2001) J. Biol. Chem. 276:28106.
- Forgione, R. et al. (2020) iScience. 23:101231.
- Whitney, G. et al. (2001) Eur. J. Biochem. 268:6083.
- Yousef, G.M. et al. (2001) Biochem. Biophys. Res. Commun. 284:900.
- Munday, J. et al. (2001) Biochem. J. 355:489.
- Kitzig, F. et al. (2002) Biochem. Biophys. Res. Commun. 296:355.
- Aizawa, H. et al. (2003) Genomics 82:521.
- Rapoport, E. et al. (2003) Bioorg. Med. Chem. Lett. 13:675.
- Toh, B.-H. et al. (2013) Cell. Mol. Immunol. 10:379.
- Bandala-Sanchez, E. et al. (2013) Nat. Immunol. 14:741.
Long Name
Sialic Acid Binding Ig-like Lectin 10
Alternate Names
Siglec10, SLG2
Entrez Gene IDs
89790 (Human)
Gene Symbol
SIGLEC10
UniProt
Additional Siglec-10 Products
Product Documents for Recombinant Human Siglec-10 Fc Chimera Avi-tag Protein, CF
Product Specific Notices for Recombinant Human Siglec-10 Fc Chimera Avi-tag Protein, CF
For research use only
Loading...
Loading...
Loading...